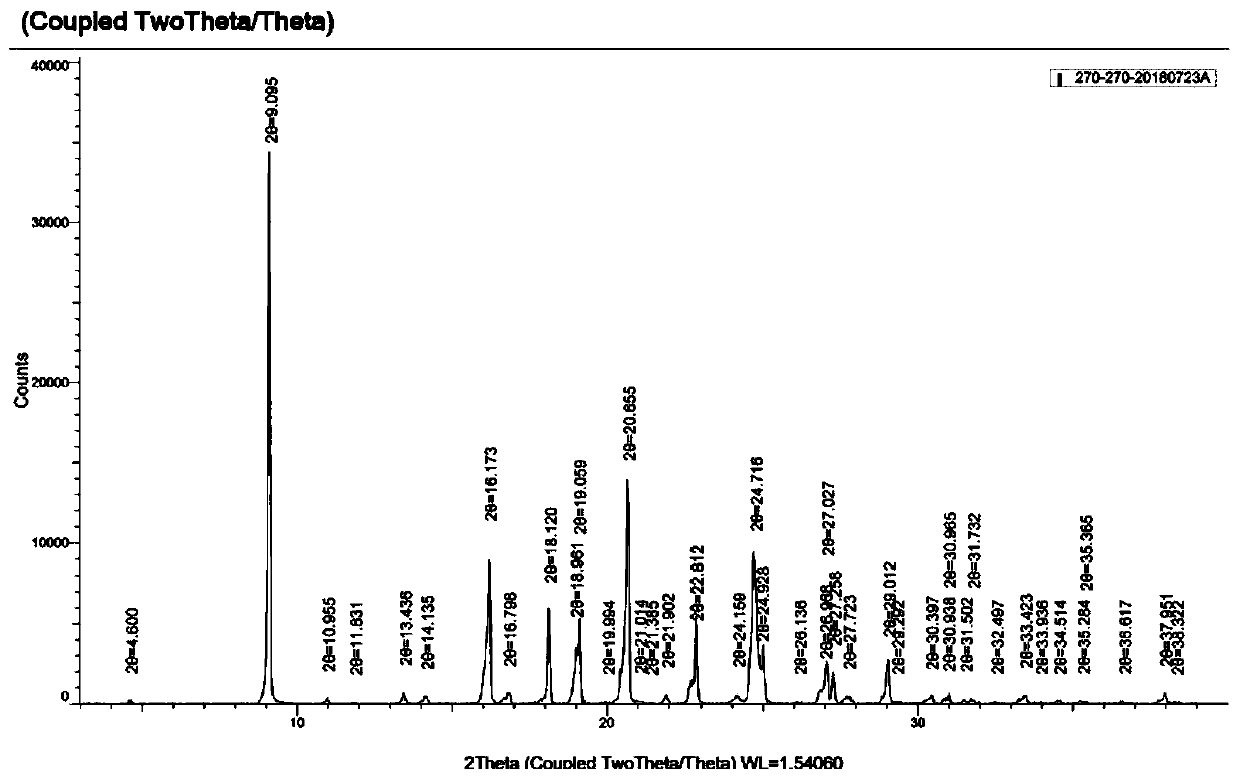
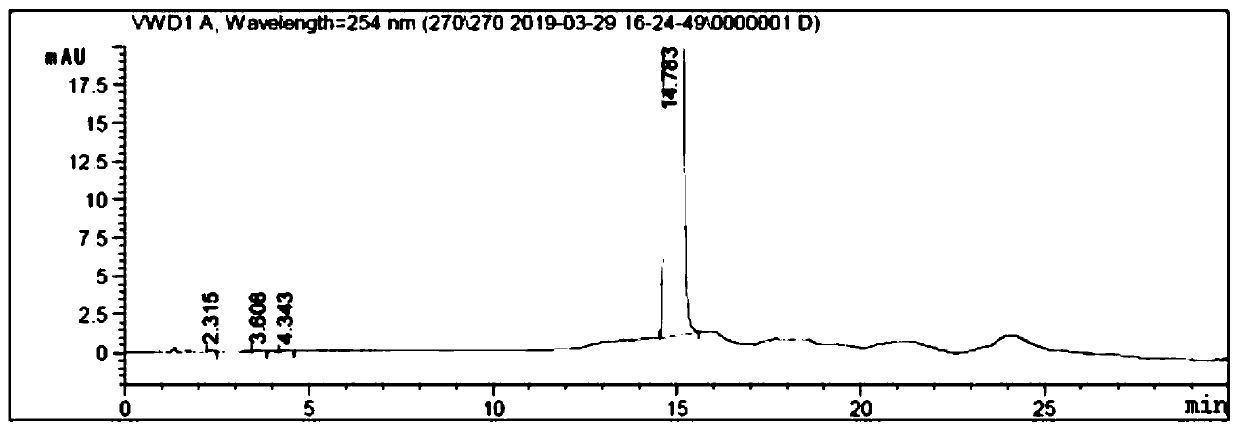
Preparation method for p-phenylbutoxybenzoic acid
A technology of phenylbutoxybenzoic acid and styrenebutene, which is applied in the field of preparation of p-phenylbutoxybenzoic acid, can solve the problems of short reaction steps and pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a preparation method of p-phenylbutoxybenzoic acid, comprising the following steps:
[0030] Under nitrogen protection, halogenated benzene, 4-halogenated-1-butene, alkali, palladium-based catalyst and the first organic solvent are mixed to carry out Heck reaction to obtain 4-halogenated-1-phenylbutene;
[0031] Under nitrogen protection, after mixing the 4-halogenated-1-phenylbutene, the first catalyst and the second solvent, the nitrogen is replaced with hydrogen to carry out catalytic hydrogenation reduction to obtain 1-halogenated phenylbutane;
[0032] Mixing the 1-halogenated phenylbutane, methylparaben, an alkaline substance and a third solvent, and carrying out a substitution reaction to obtain methyl p-phenylbutoxybenzoate;
[0033] The methyl p-phenylbutoxybenzoate, the alkaline substance and the fourth solvent are mixed to carry out a hydrolysis reaction, and then the pH value is adjusted to be acidic to obtain p-phenylbutoxybenzoic aci...
Embodiment 1
[0071] Add 500mL of toluene, 56.3g (1.00eq) of chlorobenzene, 54.38g (1.20eq) of 4-chloro-1-butene, 90.9g (1.8eq), catalyst Pd (PPh) to a 1L four-necked bottle 3 ) 4 (1%), replaced with nitrogen three times, heated to 100° C., the incubation reaction was completed for 7h, and the chlorobenzene reaction was completed by GC detection (GC detection was less than 0.1%). Cool to 25°C, add 500 mL of water, filter, take the filtrate and wash it with 300 mL of water three times, take the organic phase and concentrate to obtain 79.2 g of 4-chloro-1-phenylbutene; the yield is 95%.
[0072] Add 300mL of ethanol, 300mL of water, 100g of 4-chloro-1-phenylbutene and 3g of 10% dry palladium carbon successively in the 1L four-necked flask, replace with hydrogen three times, and 0.3Bar reacts completely in 8 hours (GC detects 4-chloro-1- phenylbutene is less than 0.1%), stop the reaction, filter, and concentrate the filtrate to obtain 88.9 g of liquid, namely the product 1-chlorophenylbutane,...
Embodiment 2
[0079] Add 100mL of toluene, 157g (1.00eq) of bromobenzene, 108.66g (1.20eq) of 4-chloro-1-butene, 181g (1.8eq), catalyst Pd (PPh) to a 2L four-necked bottle 3 ) 4 (1%), replaced with nitrogen three times, heated to 90° C., the incubation reaction was completed for 8h, and the bromobenzene reaction was completed by GC detection (GC detection was less than 0.1%). Cool to 25°C, add 500 mL of water, filter, take the filtrate and wash with 300 mL of water three times, take the organic phase and concentrate to obtain 157.3 g, 4-chloro-1-phenylbutene, with a yield of 95%.
[0080]In the 1L four-necked flask, add ethanol 300mL, water 300mL, 4-chloro-1-phenylbutene 100g and 3g 5% dry palladium carbon successively, hydrogen is replaced three times, and 0.3Bar reacts completely in 8 hours (GC detects 4-chloro-1 - phenylbutene is less than 0.1%), stop the reaction, filter, and concentrate the filtrate to obtain 87 grams of liquid, namely the product 1-chlorophenylbutane, with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com