Synthesis method of ulipristal acetate
A technology of ulipristal acetate and a synthetic method, which is applied in the direction of steroids, organic chemistry, etc., can solve the problems that cannot meet the requirements of residual solvents and limits, and achieve the effect of high product yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
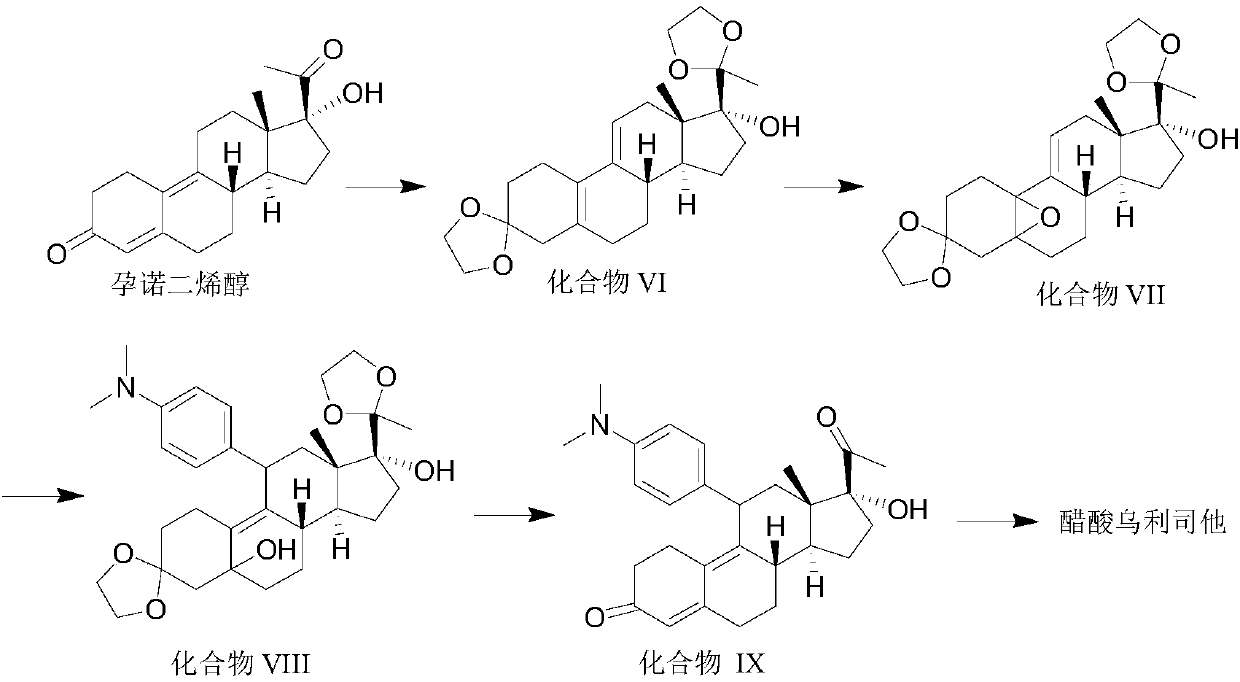
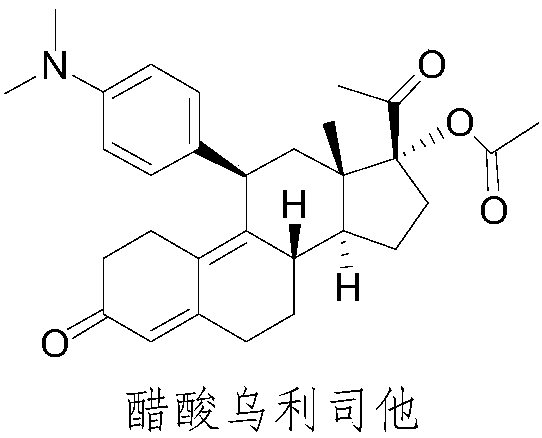
[0033] The invention provides a synthetic method of ulipristal acetate, which uses commercially available pregnanodienol as a starting material, and undergoes keto group protection, oxidation, Grignard addition, hydrolysis and esterification in sequence to finally obtain acetic acid Ulistat. In this method, the reaction conditions for obtaining ulipristal acetate from ulipristal are relatively mild, and it is difficult to produce demethylated ulipristal or nitric oxide ulipristal, and the final ulipristal has high purity.
[0034] A kind of synthetic method of ulipristal acetate, is characterized in that, reaction formula is as follows:
[0035]
[0036] The method comprises the steps of:
[0037] 1) Pregnodienol generates compound VI under the action of ethylene glycol, p-toluenesulfonic acid and trimethyl orthoformate;
[0038] This step is to protect the two ketone groups to avoid destroying the ketone groups during the next step of oxidation;
[0039] 2) compound VI ...
Embodiment 1
[0046] Embodiment 1: the preparation of compound VI
[0047] Take 62.8g (0.20mol) pregnanodienol, 80mL (1.44mol) ethylene glycol, 55mL (0.5mol) trimethyl orthoformate and dissolve in 500mL dichloromethane, add 7.6g (0.04mol) P-toluenesulfonic acid monohydrate, heat up to 35°C, stir the reaction until TLC shows that the reaction of pregnodienol is complete, add saturated aqueous sodium bicarbonate solution to neutralize, separate the organic phase, extract the aqueous phase with 300mL dichloromethane each time, and dry , add 0.5mL of pyridine, distill under reduced pressure to obtain a thick substance, add 300mL of methanol to the thick substance, stir at 0-5°C for 45min, filter, wash the filter cake with ice methanol, and dry in vacuo to obtain 66.3g of compound VI (0.167 mol), yield 83%.
Embodiment 2
[0048] Embodiment 2: the preparation of compound VII
[0049]Dissolve 60.3g (0.15mol) of compound VI, 7.6mL (0.05mol) of hexachloroacetone, and 1.5mL (0.019mol) of pyridine in 200mL of dichloromethane, and add 90mL (1.5mol) of 50% hydrogen peroxide dropwise at 0-5°C , keep stirring at 0-5°C, until TLC monitors that compound VI disappears, separate the organic phase, extract the aqueous phase with 100mL of dichloromethane, combine the organic phases, quench with aqueous sodium thiosulfate until the aqueous phase is neutral, dry, reduce Concentrate under reduced pressure to obtain compound VII, which is directly used in the next reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com