Novel method for preparing penehyclidine hydrochloride
A technology of penehyclidine hydrochloride and penehyclidine, which is applied in the field of preparation of penehyclidine hydrochloride, can solve the problems of high cost, harsh conditions, and difficult purification of penehyclidine hydrochloride, and achieve the elimination of distillation Crystallization, low production cost, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
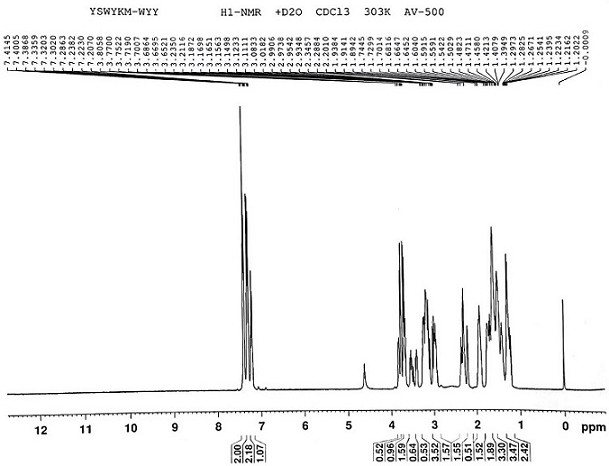
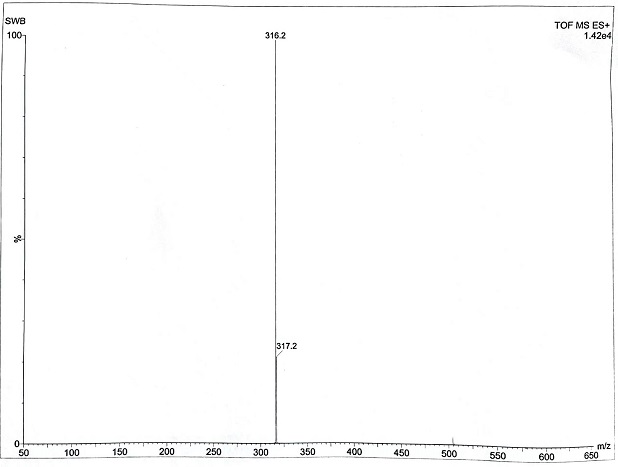
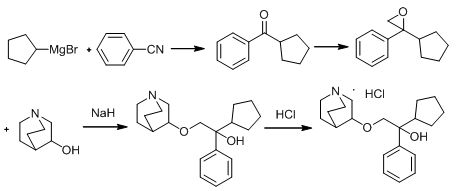
[0028] Preparation of Intermediate 1: Add quinuclidin-3-ol (50g, 0.39mol), paraformaldehyde (39.0g, 0.43mol), and 250ml dimethyl sulfoxide into a 2000ml three-necked flask in turn, stir rapidly, and pass through to dry Hydrogen chloride gas (produced by mixing concentrated sulfuric acid and sodium chloride), the temperature of the reaction solution was controlled by an ice-water bath not to exceed 40 °C, and the hydrogen chloride was passed in for 5 hours, then stopped, and nitrogen was passed into the reaction solution to purge the hydrogen chloride gas, and 750ml of water was added. , Slowly add saturated sodium bicarbonate solution dropwise to adjust pH to 8-9, extract twice with 100 ml of ethyl acetate each time, combine the organic layers, wash with 150 ml of saturated brine, dry over anhydrous magnesium sulfate, filter, and distill the filtrate under reduced pressure to dry to obtain 41.0 g of pale yellow liquid.
Embodiment 2
[0030] Preparation of intermediate 2: Dissolve 38 g of intermediate 1 in 80 ml of anhydrous tetrahydrofuran, stir evenly, add 10 ml to a 250 ml three-necked flask, add magnesium chips (6.3 g, 0.26 mol), and 2 elemental iodine, stir evenly, and react The liquid turned yellow, heated to about 45 ℃ of the reaction liquid, and began to generate bubbles, the color of the reaction liquid gradually subsided, and the reaction was initiated. The reaction was continued to be stirred for 2 h to reflux to obtain a solution of Intermediate 2, which was directly used in the next reaction.
Embodiment 3
[0032] Preparation of intermediate 3: N-methoxy-N-methylcyclopentanecarboxamide (31.4 g, 0.20 mol) and 125 ml of anhydrous tetrahydrofuran were added to a 500 ml three-necked reaction flask, respectively, and the intermediate obtained in Example 2 was added 2 The solution was added dropwise to the above solution through a constant pressure dropping funnel. During the dropwise addition, the temperature of the reaction solution was controlled not to exceed 30°C by means of an ice-water bath and the drop rate. The reaction was quenched. After the dropwise addition, saturated potassium carbonate aqueous solution was added in batches to adjust the pH to 8-9, the solution was separated, dried over anhydrous magnesium sulfate, and filtered to obtain the tetrahydrofuran solution of intermediate 3, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com