Preparation method of pridinol mesylate
A technology of predinol and methanesulfonic acid, applied in the field of preparation of predinol mesylate, can solve problems such as long route and low yield, and achieve the effects of simple post-processing, short synthesis route and reduced raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
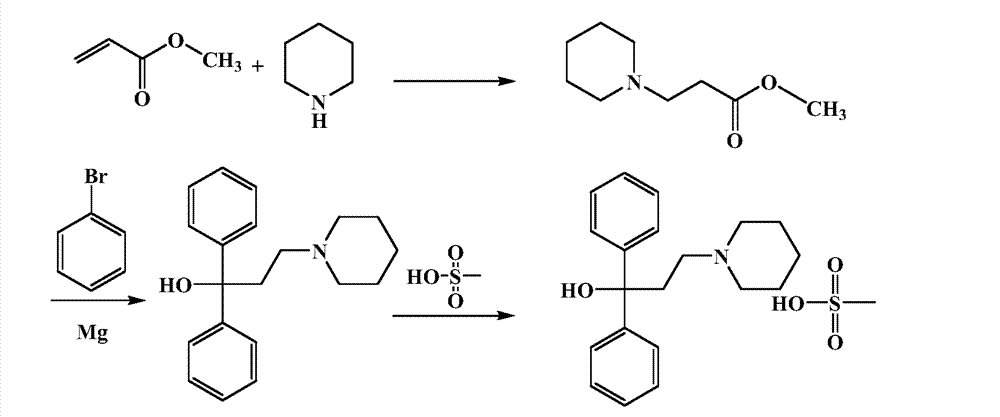
[0017] Methyl acrylate (688.72g, 8mol) was added to a 1L three-necked flask, and piperidine (681.2g, 8mol) was added to a constant pressure dropping funnel, and quickly added dropwise to the three-necked flask within 30min, and the dropwise addition was completed. Heated to 110°C, the reaction system was refluxed, and the reaction was tracked by GC. The reaction is about 2-3h, and the methyl acrylate in the reaction system disappears. Distilled under reduced pressure, and collected fractions at 72-75°C / 2mmHg to obtain methyl 3-(1-piperidinyl)propionate (1367.3 g, yield 99.81%) as a colorless transparent liquid, which was ready for use.
Embodiment 2
[0019] Magnesium (36.5 g, 1.50 mol) and a small amount of iodine were added to a three-necked flask equipped with a mechanically stirred reflux condenser and a drying tube. Bromobenzene (204.12g, 1.30mol) was added with 1L tetrahydrofuran to prepare a solution of bromobenzene in tetrahydrofuran, and the solution of bromobenzene in tetrahydrofuran was added dropwise to the reaction system to keep reflux in the system. magnesium bromide Grignard reagent.
[0020] Add 3-(1-piperidinyl)methyl propionate (85.70 g, 0.50 mol) prepared in Example 1 into a three-necked flask equipped with mechanical stirring and a drying tube, add 300 mL of tetrahydrofuran, cool to 0 ° C, and start to drop After the addition of phenylmagnesium bromide Grignard reagent was completed, the temperature was raised to 66°C, and the reaction was stirred under reflux for 3h. Then the reaction system was cooled to 0° C., and 1 L of 4M HCl solution was added dropwise for hydrolysis. After the hydrolysis is com...
Embodiment 3
[0022] Add pridinol (110.21 g, 0.37 mol) and 600 ml of diethyl ether into a 1 L three-necked flask, and cool to -15°C. Add 200ml of ether to methanesulfonic acid (53.35g, 0.56mol) to make methanesulfonic acid ether solution, add dropwise the methanesulfonic acid ether solution into the three-necked flask, during the dropping process, a white solid will precipitate out, keep stirring for 30min after dropping . After the reaction was completed, it was filtered, the filter cake was washed with 2×200ml ether, and dried in vacuum at 70° C. to obtain a white solid of pritinol mesylate (124.7 g, yield 86.08%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com