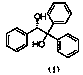
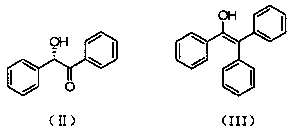
Preparation method of (S)-(-)-1,1,2-triphenyl-1,2-ethanediol
A technology of ethylene glycol and triphenyl glycol, which is applied in the field of preparation of 1,1,2-triphenyl-1,2-ethylene glycol, can solve the problems of incomplete reaction, limited mixing effect of the reactor, and poor yield. In order to achieve the effect of precise temperature control, conversion rate and product selectivity improvement, and reaction efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
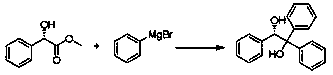
[0018] Embodiment 1, after methyl mandelate and phenylmagnesium bromide are respectively dissolved in 2-methyltetrahydrofuran, the molar concentration is 0.5mol / L methyl mandelate solution and the molar concentration is 0.5mol / L phenyl bromide Magnesium solution; The methyl mandelate solution and the phenylmagnesium bromide solution that are made are imported into the first microchannel reactor by a metering pump and carry out the main reaction respectively, and the reaction solution obtained after the reaction directly flows into the second microchannel reactor, When the reaction solution flows into the second microchannel reactor, the hydrochloric acid aqueous solution is input into the second microchannel reactor through a metering pump to carry out the quenching reaction, and the reaction solution is obtained after the quenching reaction, and the reaction solution flows out in a state of continuous flow of highly dispersed phase To the receiver, separate the reaction liquid...
Embodiment 2
[0019] Embodiment 2, after methyl mandelate and phenylmagnesium bromide are respectively dissolved in 2-methyltetrahydrofuran, the obtained molar concentration is 0.5mol / L methyl mandelate solution and the molar concentration is 0.5mol / L phenyl bromide Magnesium solution; The methyl mandelate solution and the phenylmagnesium bromide solution that are made are imported into the first microchannel reactor by a metering pump and carry out the main reaction respectively, and the reaction solution obtained after the reaction directly flows into the second microchannel reactor, When the reaction solution flows into the second microchannel reactor, the sulfuric acid aqueous solution is input into the second microchannel reactor through a metering pump to carry out the quenching reaction, and the reaction solution is obtained after the quenching reaction is completed, and the reaction solution flows out in the state of continuous flow of highly dispersed phase To the receiver, separate...
Embodiment 3
[0020] Embodiment 3, after methyl mandelate and phenylmagnesium bromide are dissolved in 2-methyltetrahydrofuran respectively, it is 1mol / L methyl mandelate solution and molar concentration that 1mol / L phenylmagnesium bromide solution is obtained The obtained methyl mandelate solution and the phenylmagnesium bromide solution are input into the first microchannel reactor by a metering pump and carry out the main reaction respectively, and the reaction solution obtained after the reaction directly flows into the second microchannel reactor, When the solution flows into the second microchannel reactor, the nitric acid aqueous solution is input into the second microchannel reactor through a metering pump to carry out the quenching reaction. After the quenching reaction is completed, the reaction solution is obtained, and the reaction solution flows out to the receiver in the state of continuous flow of highly dispersed phase. In the container, the reaction liquid in the receiver wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com