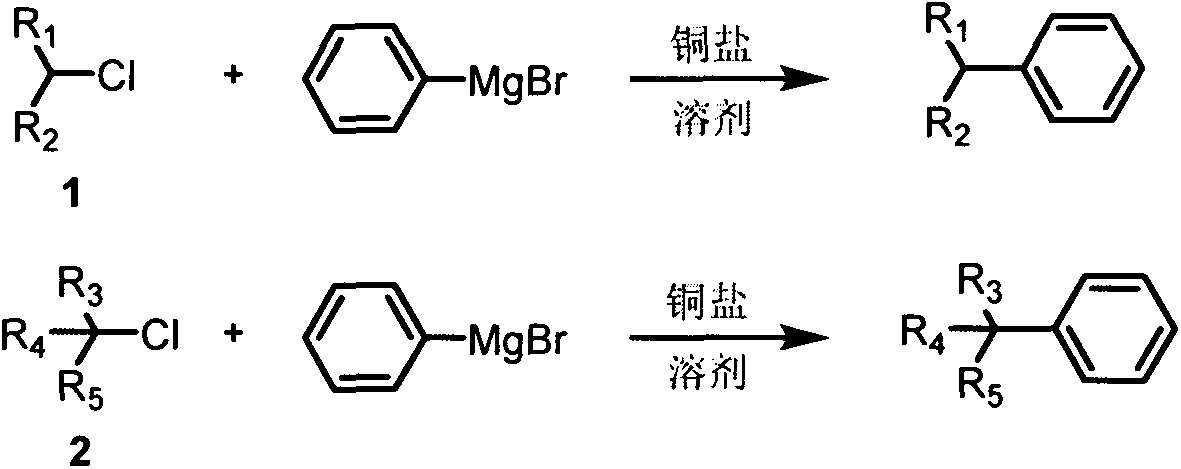
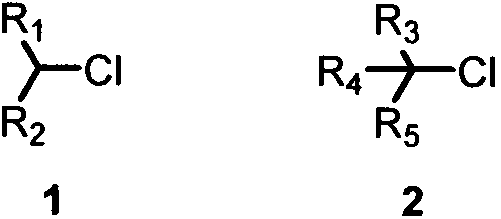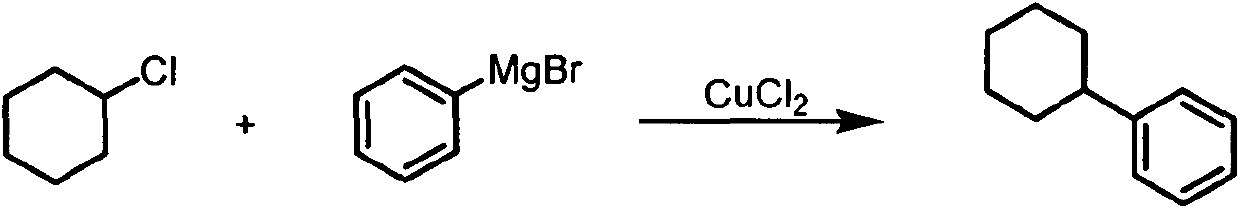Cross-coupling method of alkyl chloride and phenyl magnesium bromide
A technology of phenylmagnesium bromide and alkyl chloride, used in copper salt catalyzed inactive secondary, copper salt catalyzed cross-coupling of alkyl chloride and phenylmagnesium bromide, tertiary alkyl chloride and phenylmagnesium bromide. In the field of cross-coupling of phenylmagnesium bromide, it can solve the problems such as the difficulty of realizing-carbon cross-coupling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Cross-Coupling of Cyclohexyl Chloride and Phenylmagnesium Bromide
[0018]
[0019] Add 13 mg (0.1 mmol) copper chloride to the Schlenk reaction tube, use the Schlenk double row tube to evacuate, and pass in argon, repeating three times. Under the state of argon, add 118uL (1.0mmol) chlorocyclohexane, 2.0mL of 2-methyltetrahydrofuran, 2.2mL of phenylmagnesium bromide (2.2mmol, 1.0mol / L, 2-methyltetrahydrofuran solution) , seal the reaction system, put the reaction tube into an oil bath at 80°C, stir and react for 12 hours, add 3mL dilute hydrochloric acid (concentration 1mol / L) and 10mL petroleum ether (boiling range 30-60°C), stir for 10 minutes, Extract and separate liquids, dry over anhydrous magnesium sulfate, and filter. After concentration, the obtained filtrate is separated and purified by silica gel column chromatography, and the eluent is petroleum ether (boiling range: 30-60° C.), and the yield is 63%. 1 H NMR (500MHz, CDCl 3 )δ7.31-7.24 (m, 2H...
Embodiment 2
[0020] Example 2: Cross-Coupling of Cyclopentyl Chloride and Phenylmagnesium Bromide
[0021]
[0022] Add 19 mg (0.1 mmol) of cuprous iodide to the Schlenk reaction tube, use the Schlenk double row tube to evacuate, and pass in argon, and repeat three times. Under argon atmosphere, add 104uL (1.0mmol) cyclopentyl chloride, 2.0mL n-hexane, 1.0mL phenylmagnesium bromide (2.0mmol, 2.0mol / L, 2-methyltetrahydrofuran solution), seal the reaction system , put the reaction tube in an oil bath at 80°C, stir and react for 14 hours, add 3mL dilute hydrochloric acid (concentration 1mol / L) and 10mL petroleum ether (boiling range 30-60°C), stir for 10 minutes, extract and separate the liquid, Dry over anhydrous magnesium sulfate and filter. After concentration, the obtained filtrate is separated and purified by silica gel column chromatography, and the eluent is petroleum ether (boiling range: 30-60° C.). The yield is 70%. 1 H NMR (500MHz, CDCl 3 )δ7.30-7.22 (m, 4H), 7.19-7.14 (m, 1H)...
Embodiment 3
[0023] Example 3: Cross-coupling of 1-phenyl-3-chlorobutane with phenylmagnesium bromide
[0024]
[0025] Add 19 mg (0.1 mmol) of cuprous iodide to the Schlenk reaction tube, use the Schlenk double row tube to evacuate, and pass in argon, and repeat three times. Under the state of argon, add 168uL (1.0mmol) 1-phenyl-3-chlorobutane, 2.0mL of toluene, 2.4mL phenylmagnesium bromide (2.4mmol, 1.0mol / L, 2-methyltetrahydrofuran solution ), seal the reaction system, put the reaction tube into an oil bath at 80°C, stir and react for 12 hours, add 3mL dilute hydrochloric acid (concentration 1mol / L) and 10mL petroleum ether (boiling range 30-60°C), and stir for 10 minutes , extracted and separated, dried over anhydrous magnesium sulfate, filtered, and the resulting filtrate was concentrated and separated by silica gel column chromatography, eluting with petroleum ether (boiling range 30-60° C.), and the yield was 74%. 1 H NMR (500MHz, CDCl 3 )δ7.35-7.27(m, 2H), 7.25(t, J=7.5Hz, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com