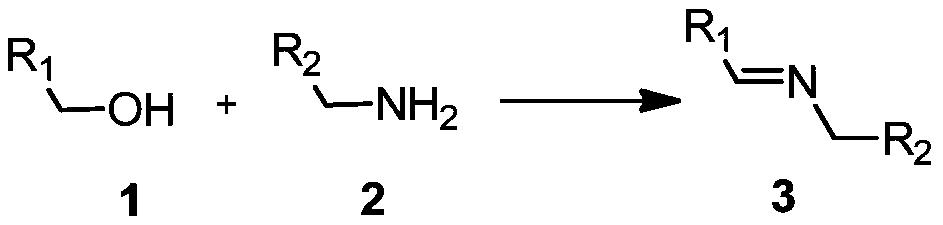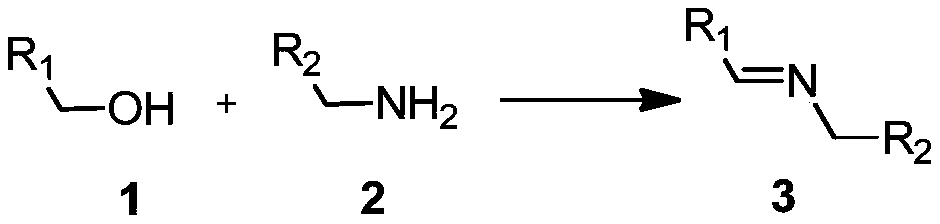Synthesis of imines by hydroxylated o-phenanthroline copper complexes/O2-catalyzed oxidation of alcohols and cross-coupling of amines
A technology of amine cross-coupling and roline copper is applied in the field of alcohol and amine cross-coupling to synthesize imines, which can solve the problems of limiting final application and long reaction time, avoiding the use of nitroxide free radicals and bases, and achieving reaction selectivity. High and wide range of substrate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
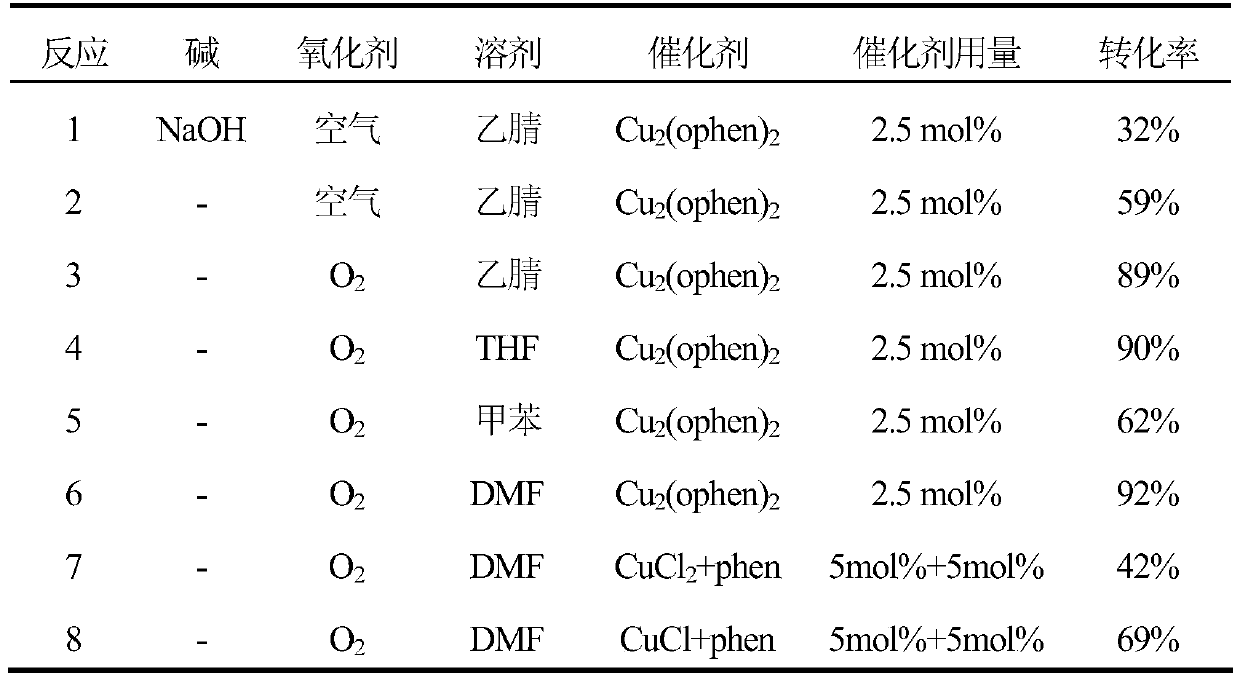
[0017] Add 50μL (0.5mmol) benzyl alcohol, 65μL (0.6mmol) benzidine, 0.0065g (0.0125mmol) Cu 2 (ophen) 2 , 2mL DMF were sequentially added to a 10mL round-bottomed flask, in a standard atmospheric pressure O 2 Reaction for 12 hours in the atmosphere at room temperature. Detected by gas chromatography (HP-5 capillary column, using nitrogen as carrier gas flow rate is 1.0mL / min, column temperature: initial temperature 40℃ (maintain 4min), increase temperature at 20℃ / min to 285℃ (maintain 2min), enter Sample port temperature: 270°C, detector temperature: 300°C), the conversion rate of benzyl alcohol was 92%, and the yield of the product N-benzylbenzaldehyde imine was 90%.
Embodiment 2
[0019] In Example 1, the benzyl alcohol used was replaced with equimolar 4-methylthiobenzyl alcohol, and the other steps were the same as in Example 1. The conversion rate of 4-methylthiobenzyl alcohol was 99% by gas chromatography. The yield of N-benzyl-4-methylthiobenzaldehyde imine was 97%.
Embodiment 3
[0021] In Example 1, the benzyl alcohol used was replaced with an equimolar 4-methoxybenzyl alcohol, and the other steps were the same as in Example 1. The conversion rate of 4-methoxybenzyl alcohol was 89% by gas chromatography. The yield of N-benzyl-4-methoxybenzaldehyde imine was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com