Synthesis method of 9,9'-spirobifluorene derivative
A technology of spirobifluorene and its derivatives, which is applied in the field of organic chemical synthesis, can solve the problems of 2-bromobiphenyl being expensive, affecting potential applications, and difficult to industrialize production, so as to meet the needs of industrialized production and achieve low cost and cost reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
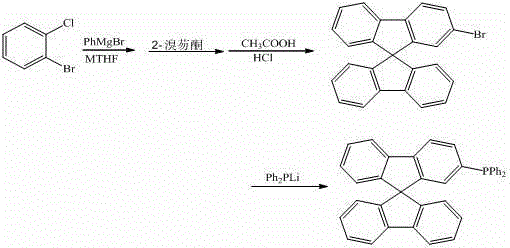
[0017]
[0018] Under the protection of argon, the reaction temperature was controlled at 100 °C, and 10 mL of o-bromochlorobenzene (0.1 mmol) in methyl tetrahydrofuran was dropped into 5 mL of phenylmagnesium bromide (0.15 mmol) in methyl tetrahydrofuran, and the reaction was stirred for 20 h. Then drop the above reaction solution into 10 mL of 2-bromofluorenone (0.1 mmol) ether solution, reflux for 2 h, hydrolyze, filter, and react the solid in 5 mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75 °C for 4 h, After filtration, the crude solid was separated by column chromatography (200-300 mesh silica gel) using a dichloromethane-n-hexane mixed solvent as the eluent to obtain 30.0 mg of 2-bromo-9,9'-spirobifluorene, with a yield of 76.0%. The obtained 2-bromo-9,9'-spirobifluorene was reacted with lithium diphenylphosphine (0.075 mmol) in 10 mL of tetrahydrofuran solvent. After reflux for 5 h, 10 mL of methanol was added to the reaction solution, and the sol...
example 2
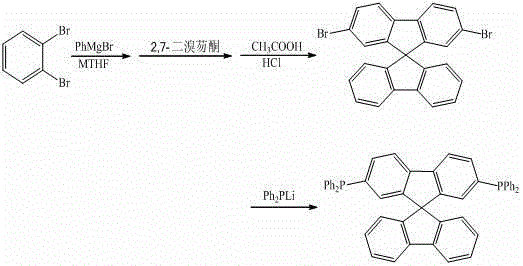
[0024]
[0025] Under argon protection, control the reaction temperature at 80 °C, drop 10 mL of o-dibromobenzene (0.1 mmol) in methyl tetrahydrofuran solution into 5 mL of phenylmagnesium bromide (0.11 mmol) in methyl tetrahydrofuran solution, and stir the reaction 12 h. Then drop the above reaction solution into 10 mL of 2,7-dibromofluorenone (0.1 mmol) ether solution, heat to reflux for 2 h, hydrolyze, filter, and dissolve the solid in 5 mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75 °C React for 4 h, filter to obtain a solid, and use dichloromethane-n-hexane mixed solvent as eluent for column chromatography (200-300 mesh silica gel) to obtain the product 2,7-dibromo-9,9'-spirobifluorene 36.2 mg , yield 76.2%. The 2,7-dibromo-9,9'-spirobifluorene obtained above was reacted with lithium diphenylphosphine (0.15 mmol) in tetrahydrofuran solvent, and after reflux for 12 h, methanol was added to the reaction solution, and the solid was obtained by filtrat...
example 3
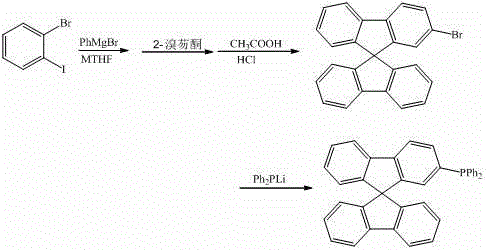
[0031]
[0032] Under argon protection, control the reaction temperature at 50 °C, drop 10 mL of o-bromoiodobenzene (0.1 mmol) in methyl tetrahydrofuran solution into 5 mL of phenylmagnesium bromide (0.09 mmol) in methyl tetrahydrofuran solution, and stir the reaction 8 h. Then drop the above reaction solution into 5 mL of 2-bromofluorenone (0.1 mol) ether solution, reflux for 2 h, hydrolyze, and filter to obtain a solid. React in 10 mL of mixed acid (glacial acetic acid and hydrochloric acid) at 75°C for 4 h, and filter , the obtained solid was separated by column chromatography (200-300 mesh silica gel) using a dichloromethane-n-hexane mixed solvent as the eluent, and dried to obtain 30.9 mg of 2-bromo-9,9'-spirobifluorene, with a yield of 78.2%. The 2-bromo-9,9'-spirobifluorene obtained above was reacted with lithium diphenylphosphine (0.075 mmol) in 5 mL of tetrahydrofuran, and after reflux for 5 h, methanol was added to obtain a solid, which was washed with water and f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com