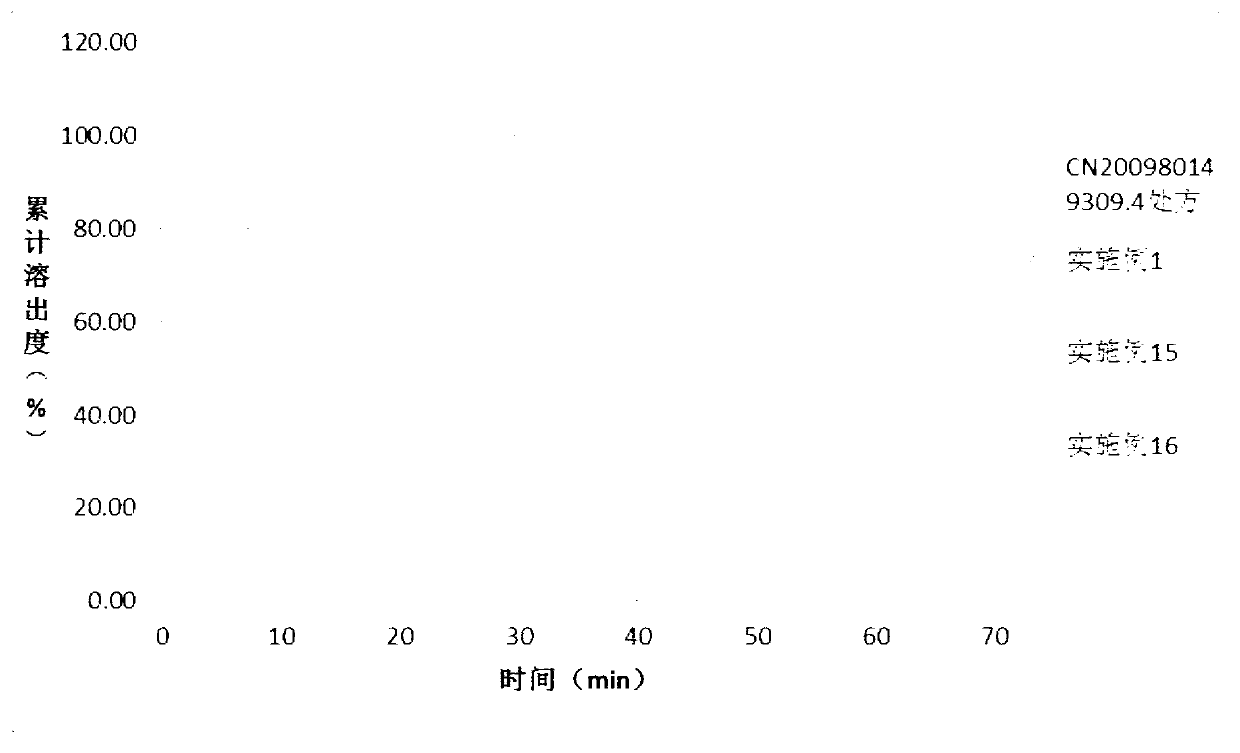
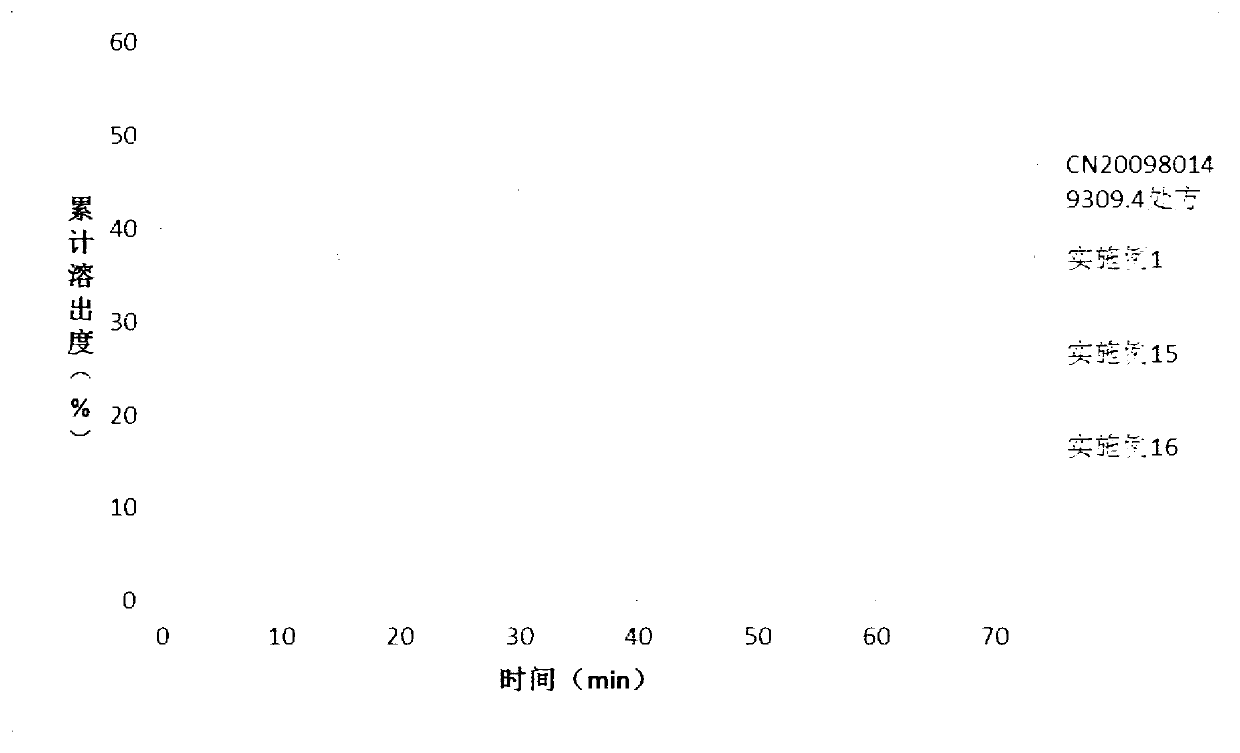
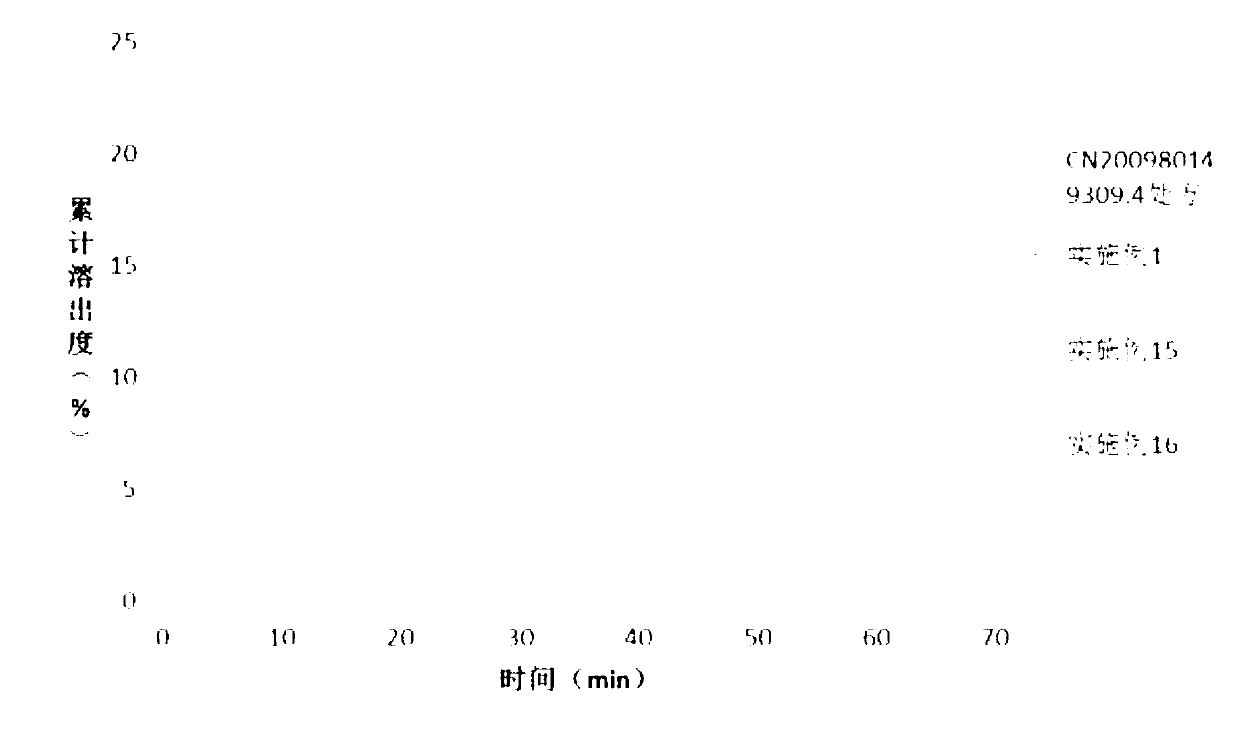
Solid dispersion and solid preparation of ulipristal acetate
A technology of ulipristal acetate and solid dispersion, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, which can solve the problem of low dissolution, achieve the best dissolution rate, and improve Dissolution amount, the effect of ensuring drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares the solid preparation of ulipristal acetate
[0022]
[0023] The preparation method is as follows: take 30 g of ulipristal acetate and 150 g of lactose, and mill them in a ball mill for 30 minutes to obtain a solid dispersion of ulipristal acetate; take 180 g of the solid dispersion, add 87 g of lactose, 15 g of povidone, and add appropriate amount of purified water Prepare into granules, add crospovidone and magnesium stearate after drying, mix well and press into tablets to obtain ulipristal acetate tablets.
[0024] The following examples are prepared for the solid dispersion of ulipristal acetate
Embodiment 2
[0026] Name of raw material Amount (g)
[0027] Ulipristal acetate 30
[0028] Lactose 90
[0029] The preparation method is as follows: take ulipristal acetate and lactose and ball mill for 45 minutes in a ball mill to obtain a solid dispersion of ulipristal acetate.
Embodiment 3
[0031] Name of raw material Amount (g)
[0032] Ulipristal acetate 30
[0033] Lactose 300
[0034] The preparation method is as follows: take ulipristal acetate and lactose and ball mill for 30 minutes in a ball mill to obtain the ulipristal acetate solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com