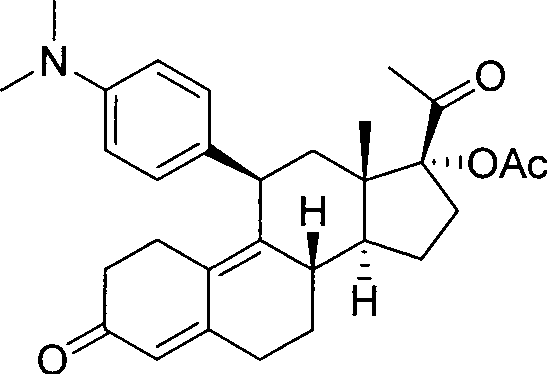
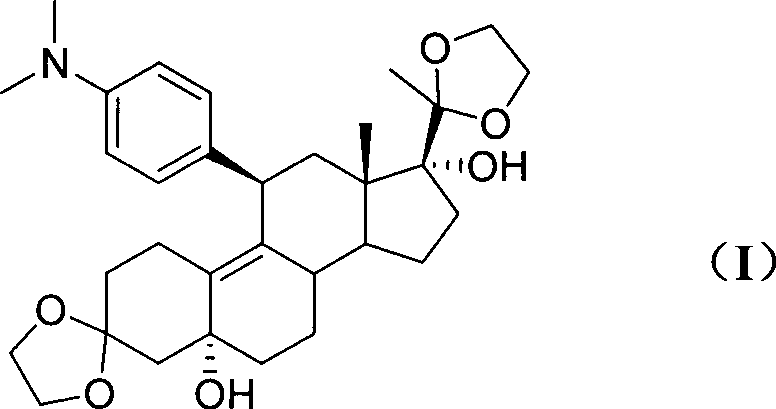
Novel preparation method of ulipristal acetate key intermediate
A technology of ethylenedioxy and norpregna, which is applied in the direction of steroids and organic chemistry, can solve the problems of unfavorable product quality, many by-products, and improvement, and achieve high yield and selectivity, and easier reaction The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
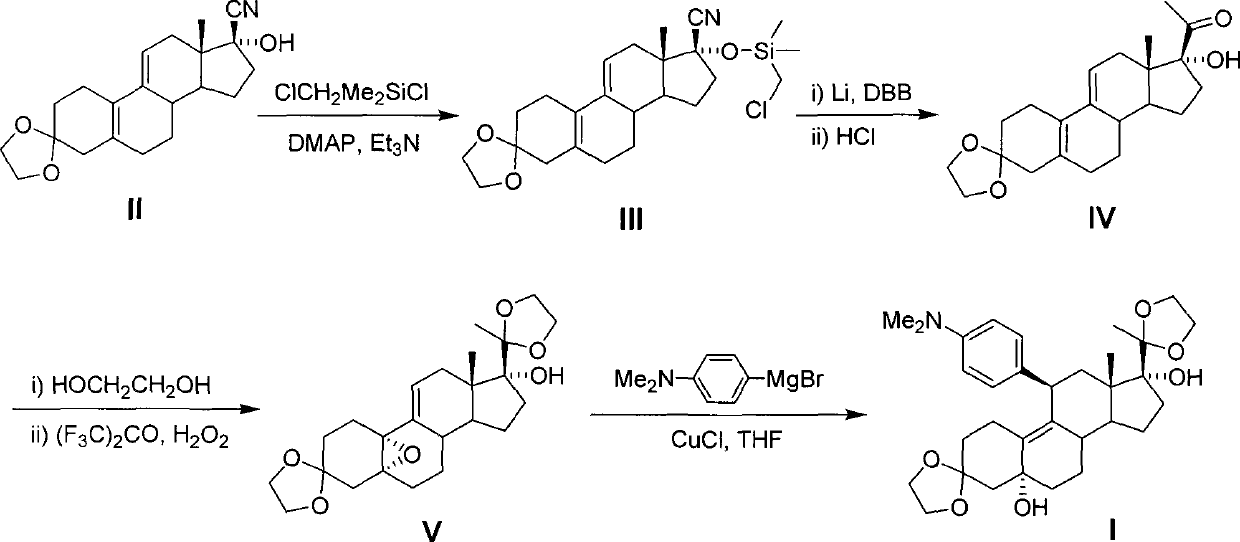
[0021] Example 1: 3,3-ethylenedioxy-17β-cyano-17α-chloromethyl (dimethyl) siloxyestradiol-5 (10), the preparation of 9 (11) diene (III )
[0022] Add 3,3-ethylenedioxy-17β-cyanoestro-5(10), 9(11)dien-17α-alcohol (II) 170.5g and 5L tetrahydrofuran into the reaction flask in sequence, and start stirring , the temperature was lowered to 5°C, 12.2 g of 4-dimethylaminopyridine and 96.5 mL of triethylamine were added, 79.0 mL of chloromethyldimethylsilyl chloride was added dropwise, and the reaction was stirred at room temperature for 12 hours. Concentrate to dryness under reduced pressure at room temperature, add 1500 mL of dichloromethane, and pour the reaction solution into 800 mL of saturated aqueous sodium bicarbonate solution while stirring. The liquid was separated, and the organic phase was concentrated to dryness under reduced pressure, 500 mL of isopropyl ether was added, filtered, and the filter cake was air-dried to obtain 219.5 g of off-white solid, with a yield of 98....
Embodiment 2
[0023] Example 2: Preparation of 17α-hydroxy-19-norpregna-4,9-diene-3,20-dione (IV)
[0024]Add 219.5 g of 3,3-ethylenedioxy-17β-cyano-17α-chloromethyl (dimethyl) siloxyestradiol-5(10), 9(11) diene (III) to In a mixed solvent of 500mL toluene and 500mL tetrahydrofuran, after stirring at room temperature for 15min, 7.0g of cuprous bromide was added and added dropwise at room temperature. After the dropwise addition, continue to stir the reaction for 23h, evaporate the solvent under reduced pressure, slowly add 300mL of 5% dilute hydrochloric acid, continue to stir at room temperature for 30min, and extract twice with dichloromethane (500mL×2), and the organic phase is sequentially washed with 300mL Wash once with saturated saline and water, dry over anhydrous magnesium sulfate, and filter. The filtrate is concentrated to dryness to obtain 142.2 g of the product, with a yield of 92.3%.
Embodiment 3
[0025] Example 3: Preparation of 3,3,20,20-bis(ethylenedioxy)-17α-hydroxyl-5α, 10α-epoxy-19-norpregna-9(11)-ene (V)
[0026] Mix 17α-hydroxy-19-norpregna-4,9-diene-3,20-dione 138.7g (IV), 122.9mL ethylene glycol and 1500mL dichloromethane, start stirring, add dropwise at room temperature A solution of triethyl orthoformate (220.4 mL) in 200 mL of dichloromethane. After the dropwise addition, 5.1 g of p-toluenesulfonic acid was added, and after stirring at room temperature for 18 hours, 700 mL of saturated sodium bicarbonate solution was slowly added dropwise. After dropping, the liquid was separated, and the organic phase was washed with saturated brine and 700 mL of water successively. After drying the organic phase over anhydrous magnesium sulfate, filter, add 71.2 g of hexafluoroacetone to the obtained dichloromethane filtrate, start stirring and cool to 4 ° C, dropwise add 30% hydrogen peroxide (125 mL) and disodium hydrogen phosphate (25 g) to mix After the solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com