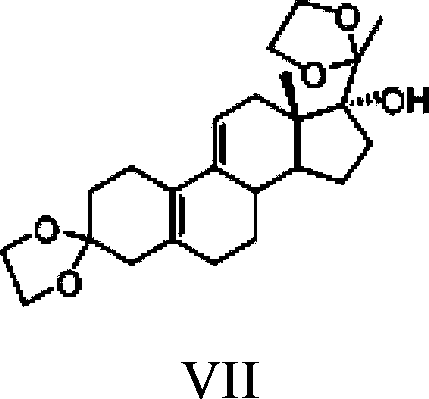
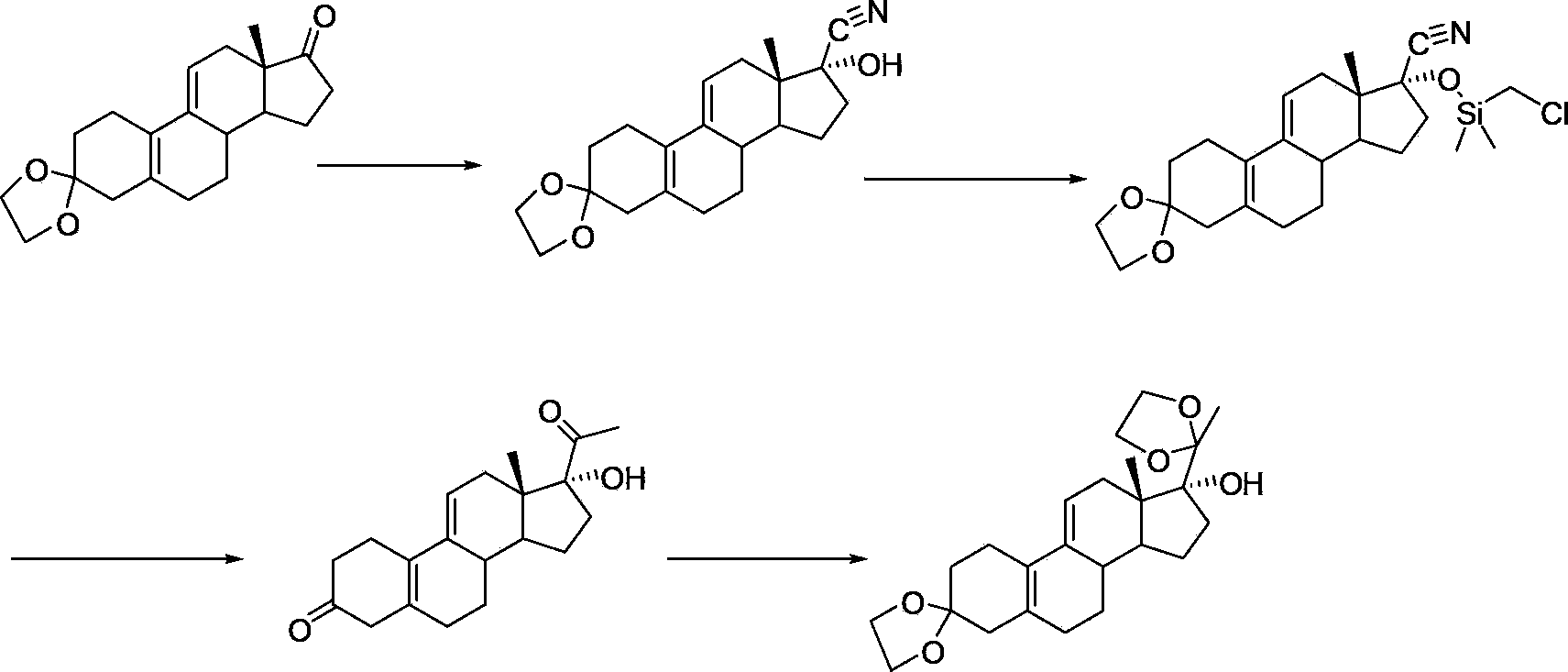
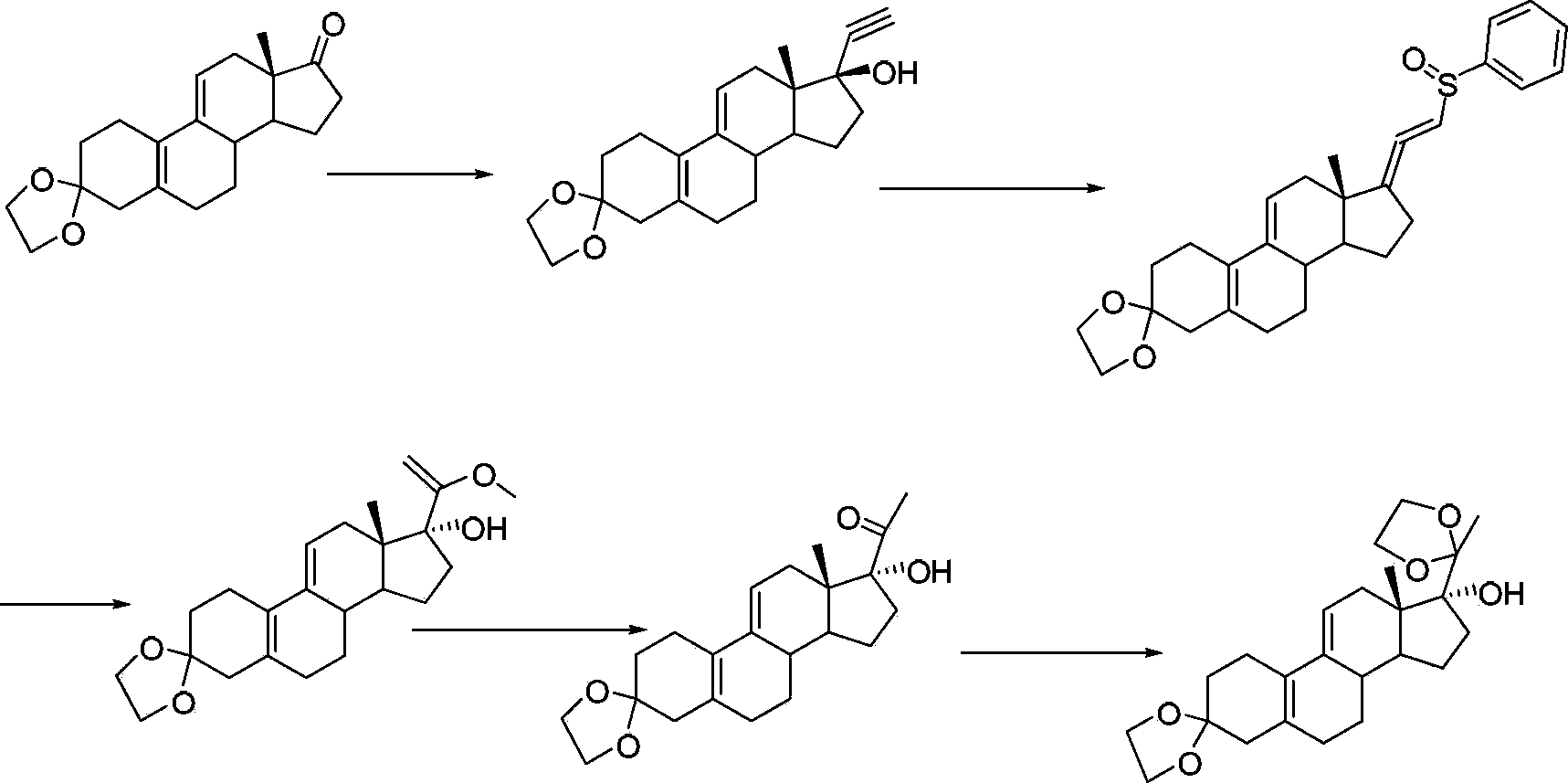
Ulipristal acetate intermediate product and preparation method thereof
A technology for hydrochloric acid and compounds, applied in the preparation of intermediates of ulipristal acetate and the field of preparation thereof, can solve the problems of low product purity, difficult product purification, many side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] The present invention provides the preparation method of the intermediate of the present invention. It should be understood that the intermediates of the present invention can also be prepared by methods or conditions commonly used in the art.
[0115] Preferably, the preparation method of the intermediate compound of formula I comprises the steps of: in an ether solvent, in the presence of hydrogen halide / ether solvent, at -20-0°C, estro-4,9-diene-3, 17-diketone and diol derivatives (HO-(CHR 1 ) n -OH, R 1 Defined as above) to react, so as to obtain the compound of formula I;
[0116]
[0117] In the formula, R is as defined above.
[0118] Wherein, the ether solvent can be selected from: ethylene glycol dimethyl ether, ethylene glycol diethyl ether, isopropyl ether, tetrahydrofuran, dioxane, or a combination thereof; the hydrogen halide / ether solvent can be Selected from: hydrogen chloride / oxane, hydrogen chloride / tetrahydrofuran, hydrogen bromide / tetrahydrofu...
Embodiment 13
[0147] Example 13, Preparation of 3-(2-methylethylenedioxy)-estro-5(10), 9(11)-dien-17-one (I-1)
[0148] Add estro-4,9-diene-3,17-dione (100.0g, 0.37mol), ethylene glycol dimethyl ether 1l, 1,2-propanediol (30.0ml, 0.41mol), 1N Hydrogen chloride in dioxane (11.0 ml) was reacted at -20°C for 4 hours. Triethylamine (200ml) was added to terminate the reaction, and the solvent was removed by concentration. The residue was dissolved in ethyl acetate, water was added for extraction, the layers were separated, and the organic phase was concentrated to dryness. The residue was crystallized from isopropyl ether, the solid was collected by filtration and dried to give the title compound (89.0 g).
[0149] MS(m / z):328.20; 1 H-NMR(DMSO):δ1.30( 3 H,18-CH 3 ),3.73-4.07( 6 H,-O-CH(CH 3 )CH 2 O-),5.50( 1 H,H-11); 13 C-NMR: 145.4(C-5), 136.8(C-9), 132.8(C-10), 124.1(C-11), 118.0(C-3), 81.5(C-17), 71.5(-O -CH 2 -), 75.2 (-O-CH-).
Embodiment 23
[0150] Example 23, Preparation of 3-(2-methylethylenedioxy)-17α-ethynyl-17β-hydroxyl-estro-5(10), 9(11)-diene (II-1)
[0151] Tetrahydrofuran (700ml) and potassium tert-butoxide (35.0g, 0.31mol) were added to the reaction flask, the temperature was lowered to -2-2°C, and acetylene gas was passed through for 15 minutes. Add I-1 (70.0 g, 0.21 mol) and continue to pass acetylene for 1 hour. After the reaction was complete, 25% ammonium chloride aqueous solution (550ml) was slowly added dropwise, and the temperature was controlled not to exceed 25°C. Ethyl acetate (300ml) was added for extraction, and the layers were separated. The organic layer was concentrated to dryness under reduced pressure to obtain the title compound.
[0152] MS(m / z):354.22; 1 H-NMR (DMSO): δ1.04 ( 3 H,18-CH 3 ),3.52(H,20-C≡CH),3.73-4.07( 6 H,-O-CH(CH 3 )CH 2 O-),5.50( 1 H,H-11); 13 C-NMR: 145.4(C-5), 136.8(C-9), 132.8(C-10), 124.1(C-11), 118.0(C-3), 87.6(C-19), 81.5(C- 17),74.0(C-20),71.5(-O-CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com