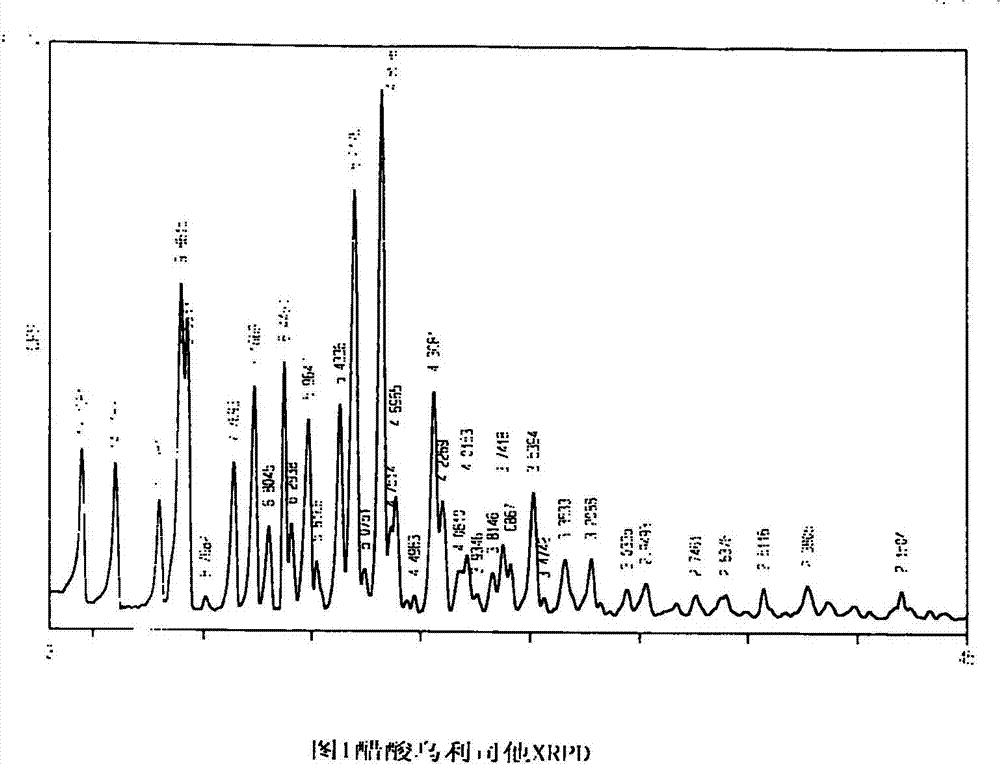
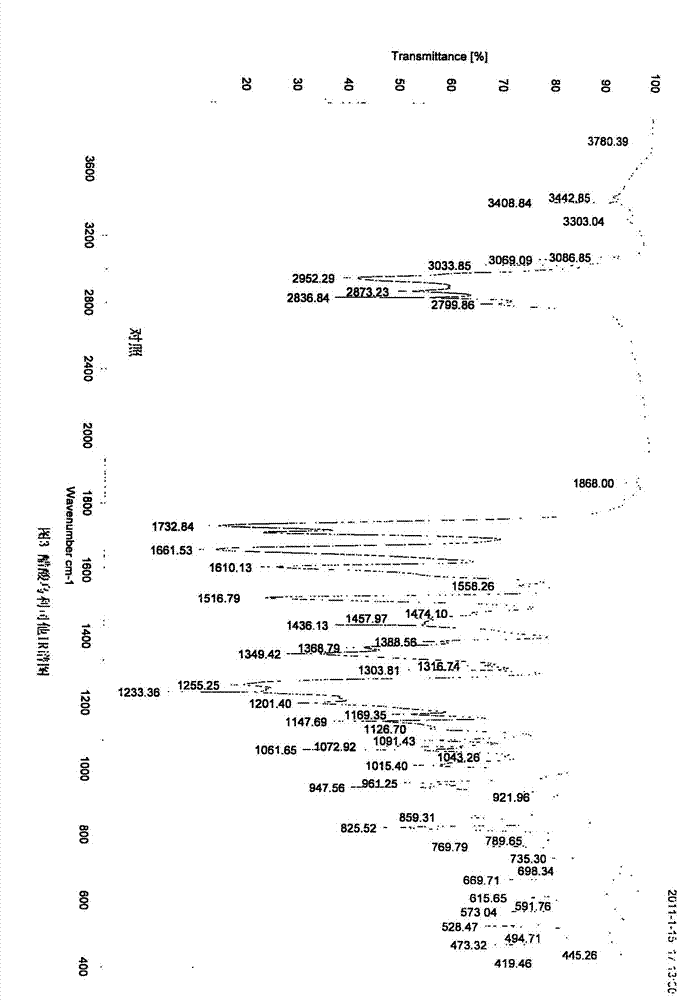
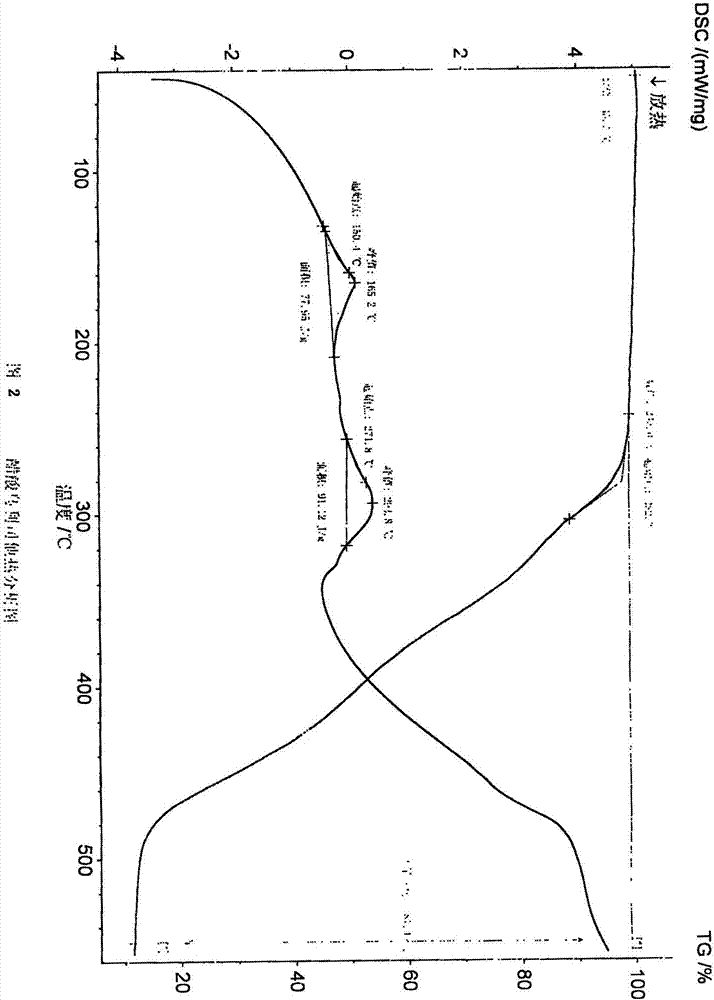
Ulipristal acetate crystals and preparation method thereof
A technology of ulipristal acetate and crystals, which is applied in the field of pharmaceuticals, can solve problems such as unsatisfactory crystal purity, and achieve the effects of good solubility, simple operation, and strong thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 25 g of crude ulipristal acetate and 50 ml of ethyl acetate to the reaction flask, heat to reflux for 30 minutes, perform hot filtration at 60°C, cool to -20°C to crystallize, and filter to obtain a white solid. Take the above white solid, add 50ml of water, stir, heat to 30°C, stir for 30 minutes, filter while hot, and dry under vacuum to obtain 20.2g of ulipristal acetate with a yield of 80.8% and a purity of HLPC of 99.75%.
Embodiment 2
[0036] Add 25 g of crude ulipristal acetate and 1250 ml of ethyl acetate to the reaction flask, heat to reflux for 10 hours, heat filter at 70°C, cool down to 0°C to crystallize, filter to obtain a white solid. Take the above white solid, add 1250ml of water, stir, heat to 50°C, stir for 30 minutes, filter while hot, and dry under vacuum to obtain 22.3g of ulipristal acetate with a yield of 89.2% and a purity of HLPC of 99.80%.
Embodiment 3
[0038] Add 25 g of crude ulipristal acetate and 500 ml of ethyl acetate to the reaction flask, heat to reflux for 5 hours, heat filter at 60°C, cool to -10°C to crystallize, filter to obtain a white solid. Take the above white solid, add 650ml of water, stir, heat to 40°C, stir for 30 minutes, filter while hot, and dry under vacuum to obtain 21.9g of ulipristal acetate with a yield of 87.6% and a purity of HLPC of 99.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com