Novel method for synthesizing ulipristal acetate
A technology of ulipristal acetate and a new method, applied in the field of chemical pharmacy, can solve the problems of large steric hindrance, low yield, long steps, etc., and achieve the effects of ingenious conception, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
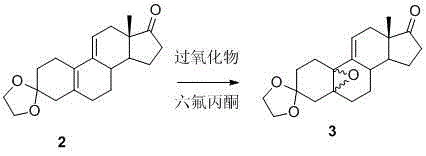
[0056] Example 1 compound 3 Synthesis
[0057]
[0058] Add 11.5g of disodium hydrogen phosphate dodecahydrate into dichloromethane, then add 8.8mL of hexafluoroacetone, 15.8mL of 30%H 2 o 2 , stirred at 0°C for 1 h, added 10 g of the compound 2 , the solution was orange-yellow, and continued to stir at 0°C for 18 hours. TLC monitored that the reaction was complete, and added 50 mL of 10% Na 2 SO 3 , stirred for 15min, the dichloromethane layer was separated, and the water layer was extracted with 2*30mL of dichloromethane, and the dichloromethane layers were combined, 10%Na 2 SO 3 (2*50mL), washed with water (2*50mL), dried with 20g of anhydrous sodium sulfate, and concentrated to give 10.6g of pale yellow foamy solid. (Theory: 10.5g yield is 100%) HPLC measured 5α, 10α / 5β, 10β as 81:19.
Embodiment 2
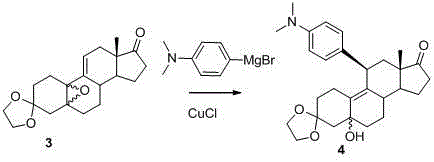
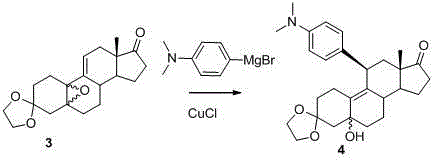
[0059] Example 2 compound 4 Synthesis
[0060]
[0061] 10.6g (0.032mol) compound 3 After dissolving with 110mL redistilled THF, add 0.076eq CuCl at -20°C and stir for 30min. If CuCl is not dissolved, slowly add 24ml (1.5eq) of 2M tetrahydrofuran solution of 4-dimethylaminophenylmagnesium bromide dropwise. , continue to stir and react at -20°C for 3 hours, TLC monitors that the reaction is complete, add 100mL of saturated ammonium chloride solution to the reaction solution to terminate the reaction, add 200mL of dichloromethane under stirring, stir for 15min, separate the dichloromethane layer and the water layer Then extract with dichloromethane 3*50mL. The organic layers were combined, washed with 3*50 mL of saturated ammonium chloride solution, dried with 45 g of anhydrous sodium sulfate, and concentrated to obtain a black oil. Placed in the refrigerator, solids precipitated, added 100 mL of isopropyl ether and stirred for 3 hours, a light blue solid precipitated, fil...
Embodiment 3
[0062] Example 3 Compound 5 Synthesis
[0063]
[0064] 10.4g (0.023mol) compound 4 Add to 100ml of dry tetrahydrofuran, add 2eq18% sodium acetylide xylene solution under ice bath, after the addition is complete, turn to room temperature and stir for 4h, TLC monitors the reaction is complete, add saturated ammonium chloride solution, spin off most of the tetrahydrofuran under reduced pressure, Add ethyl acetate to extract 3 times, 50ml each time, combine, dry over anhydrous sodium sulfate, concentrate, add 100ml of isopropyl ether, stir, a solid precipitates, filter, and dry under vacuum at 50°C for 5h to obtain 11g of light blue solid . The yield is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com