Ulipristal acetate medicine composition
A technology of unilast acetate and a composition, applied in the field of pharmaceutical preparations, can solve problems such as poor dissolution, and achieve the effects of improving solubility or dissolution, good stability, and excellent pharmaceutical technical characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
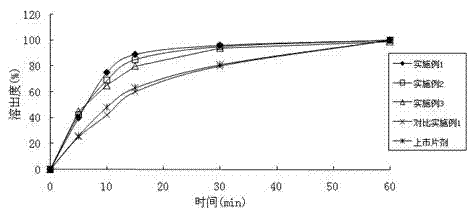
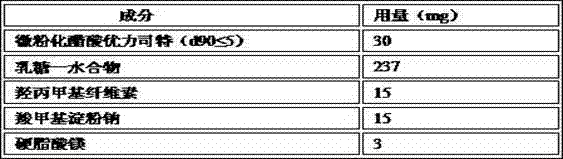
Embodiment 1
[0042] The following formulation was used for the preparation of tablets each containing 30 mg of micronized uliplast acetate.
[0043]
[0044] Manufacturing process: Tablets were prepared using standard wet granulation procedures. Sieve and mix micronized uliplast acetate (d90≤5), lactose monohydrate, and hypromellose evenly; use a high-efficiency wet mixing granulator or any other suitable wet granulator, and use purified water The mixed material is granulated; the wet granules are dried in a fluidized bed or an oven; after drying, the granules are sized; the sodium carboxymethyl starch and magnesium stearate are sieved and mixed with the dried granules; The final granules are compressed into tablets.
Embodiment 2
[0046] The following formulation was used for the preparation of tablets containing 10 mg of micronized uliplast acetate each.
[0047]
[0048]
[0049] Manufacturing process: Tablets were prepared using standard direct compression procedures. Sieve and mix micronized uliplast acetate (d90≤5), microcrystalline cellulose, hydroxypropylmethyl cellulose, and sodium carboxymethyl starch; Mix well; use a tablet machine to compress the mixture into tablets.
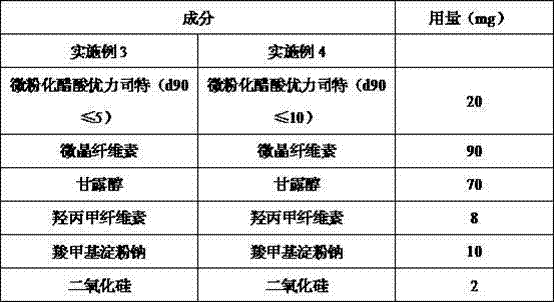
Embodiment 3-4
[0051] The following formulation is used for the preparation of hard capsules containing 20 mg of micronized uliplast acetate per tablet.
[0052]
[0053]
[0054]Preparation process: Standard wet granulation steps are used to prepare capsule granules. Sieve and mix micronized uliplast acetate (d90≤5 or d90≤10), microcrystalline cellulose, mannitol, hypromellose, and sodium carboxymethyl starch; use a high-efficiency wet mixing granulator or Any other suitable wet granulator, use purified water to granulate the mixed material; wet granules are dried in a fluidized bed or an oven; granulated after drying; sieved and mixed with the dried granules evenly; The capsule filling machine makes the mixed granules into hard capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com