Polycrystal forms of ulipristal acetate and preparation method thereof
A technology of ulipristal acetate and polymorphic forms, applied in the field of polymorphic forms of ulipristal acetate, can solve problems such as difficulty in obtaining high-purity products, helpless removal of impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
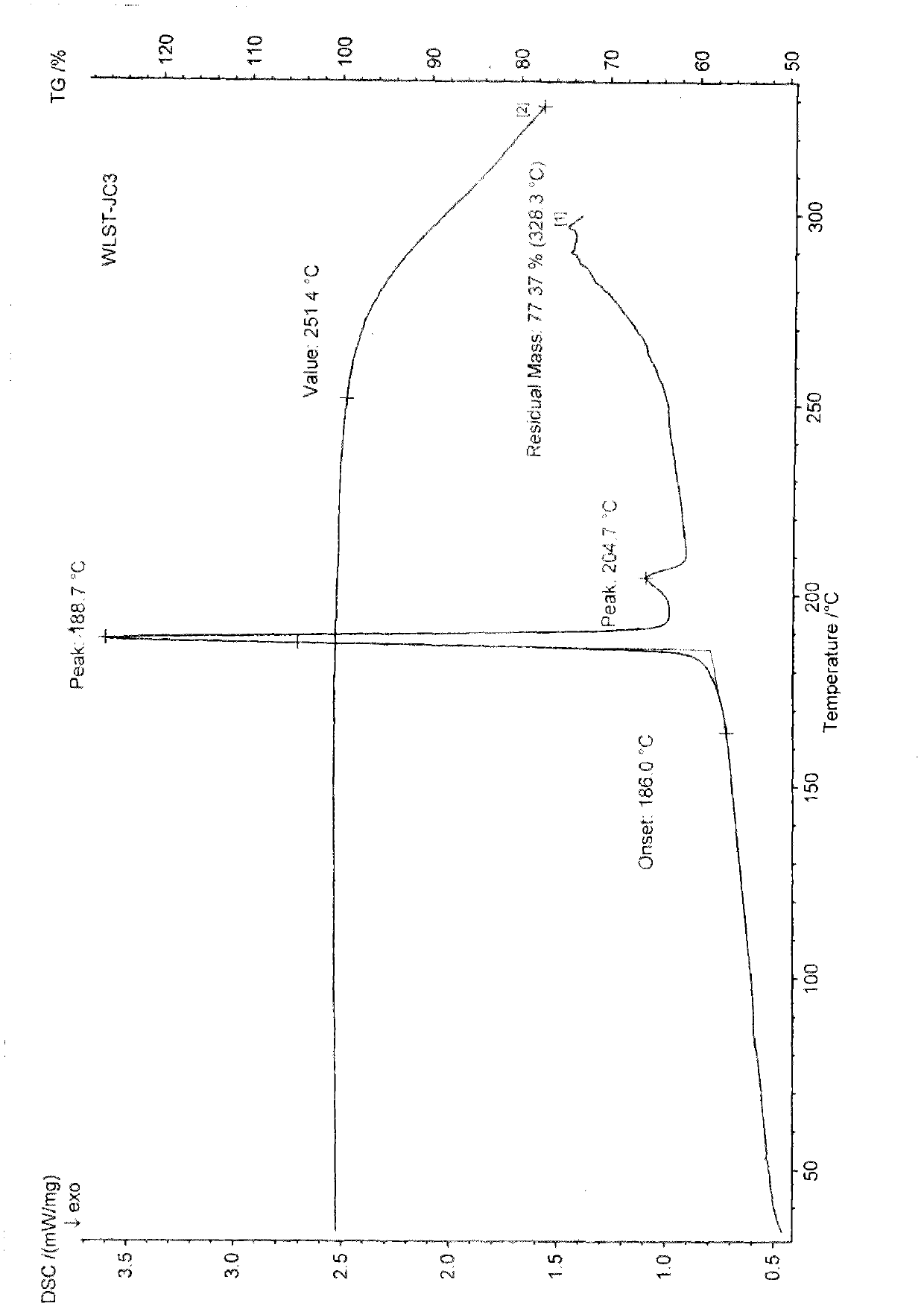
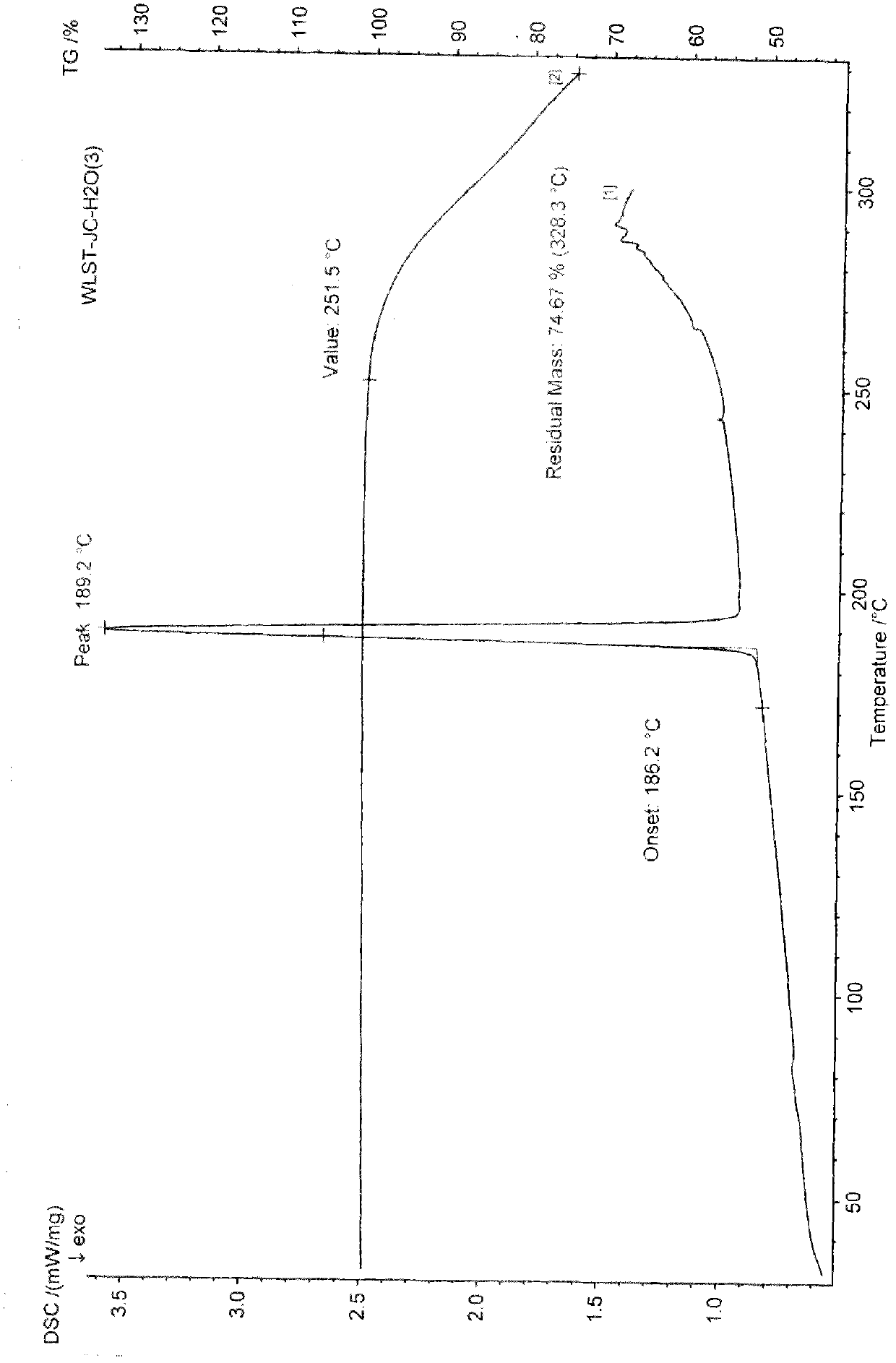
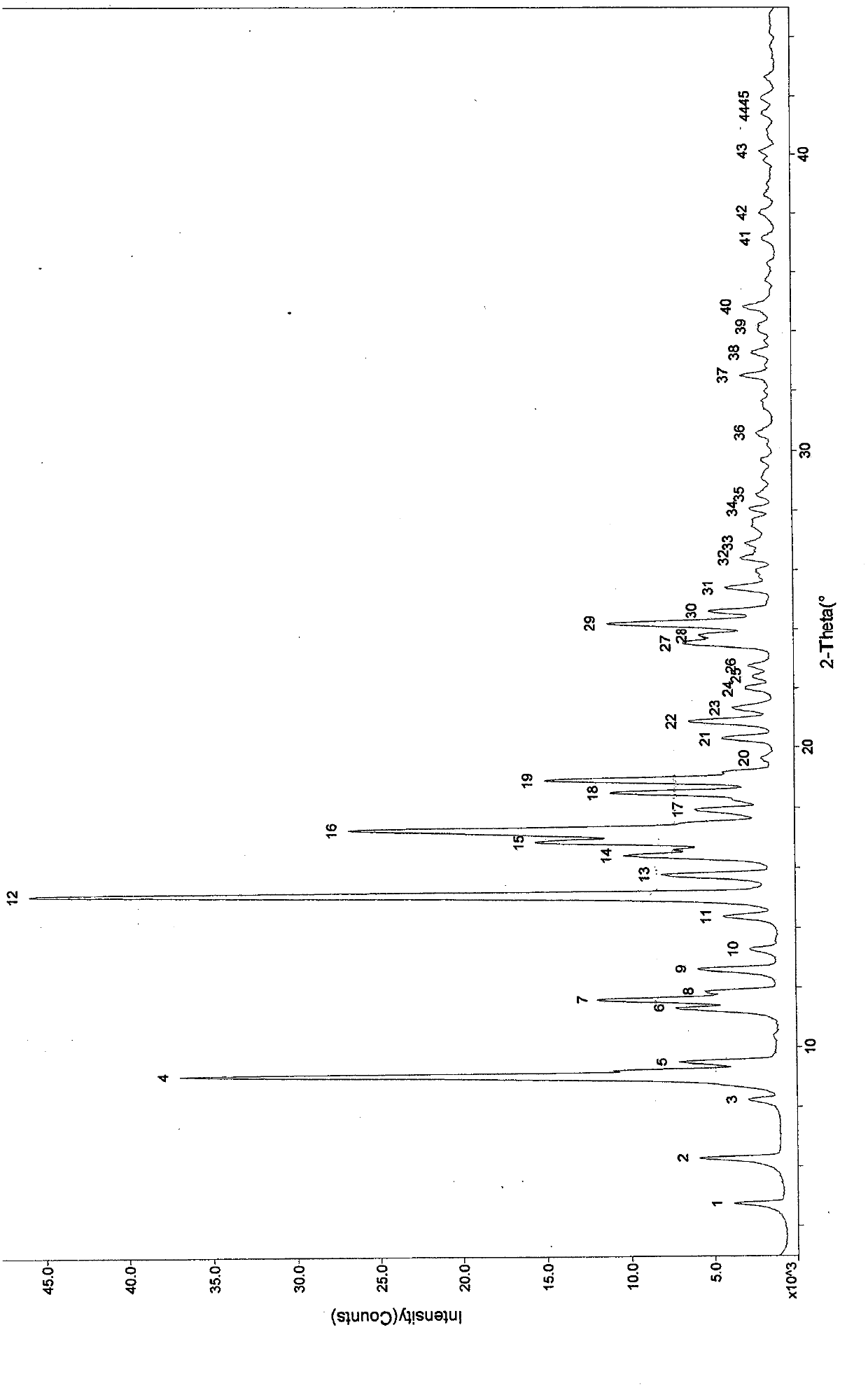
[0098] Preparation Example 1: General preparation method of ulipristal acetate solvate polymorph:
[0099] Add 1 g of ulipristal acetate crude product to 10-13 times the volume of ethanol (ml / g), heat to reflux to dissolve, add 0.1 g of activated carbon to decolorize, filter, concentrate under reduced pressure to 3-5 times the volume, and then cool to After stirring at 10°C for 30 minutes to 1 hour, filter. Dry at 60°C. Obtained 0.88 g of ulipristal acetate ethanolate, mp: 150-170°C.
[0100] Also switch to acetone, isopropanol, ethyl acetate, and crystallize to obtain different polymorphs of ulipristal acetate solvate.
preparation example 2
[0101] Preparation Example 2: Preparation method of ulipristal acetate isopropanol / ethanol solvate:
[0102] Add 1 g of crude ulipristal acetate to 10 times isopropanol / ethanol (9:1), heat to reflux to dissolve, add 0.1 g of activated carbon to decolorize, filter, then cool to 10°C while stirring, and stir for 30 minutes to 1 hour ,filter. Dry at 60°C to obtain 0.85 g of ulipristal acetate isopropanol / ethanol solvate, mp: 164-170°C.
preparation example 3
[0103] Preparation Example 3: Ulipristal acetate ethyl acetate / petroleum ether solvate preparation method:
[0104] Add 5 times of dichloromethane to dissolve 1 g of ulipristal acetate crude product, add 0.1 g of activated carbon to decolorize, filter, concentrate under reduced pressure to almost no solvent, add ethyl acetate / petroleum ether (1:5), and cool to 20°C After stirring for 30 minutes-1h, filter. Dry at 60°C to obtain 0.85 g of ulipristal acetate ethyl acetate / petroleum ether solvate, mp: 160-210°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com