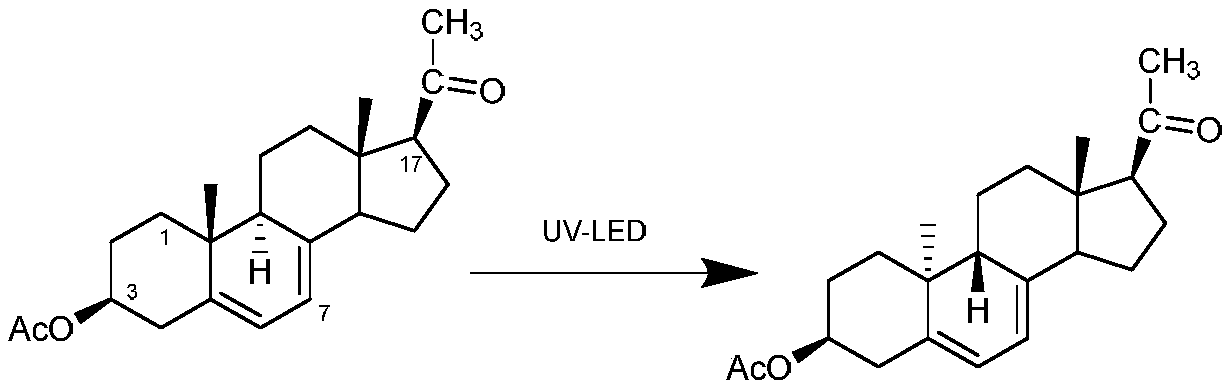
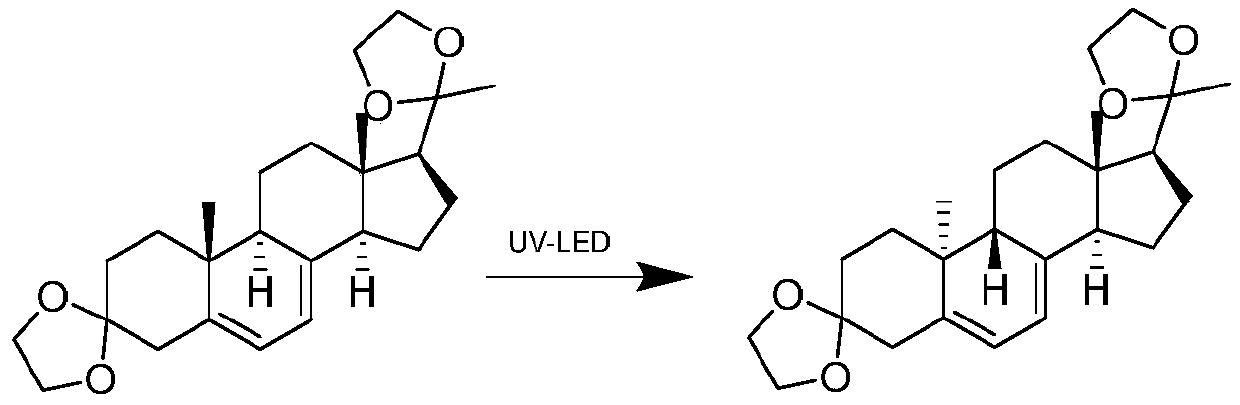
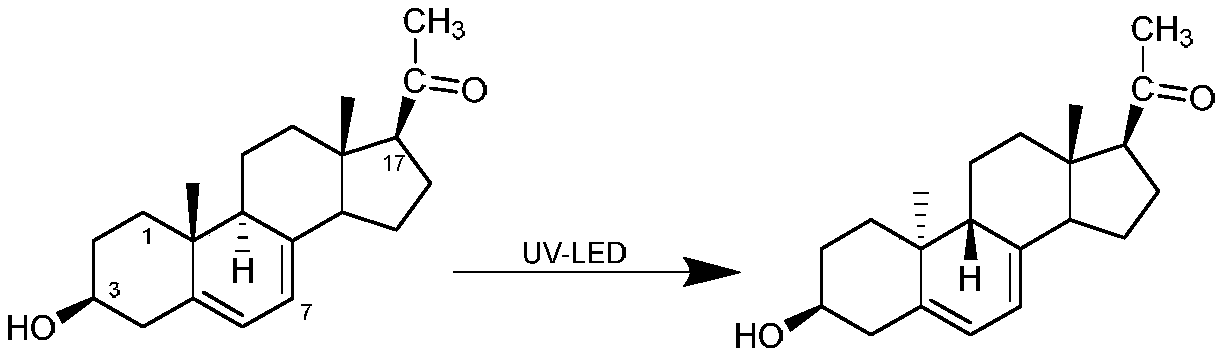
Preparation method of dydrogesterone intermediate
A technology for dydrogesterone and intermediates, which is applied in the field of dydrogesterone intermediates, can solve the problems of harsh reaction conditions, difficult to obtain raw materials, low yield and the like, and achieves high utilization rate of raw materials, shortened illumination time, and preparation process. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Uniformly mix 1 g of ergosterol and 20 mL of tetrahydrofuran to obtain solution A; irradiate solution A with an LED ultraviolet lamp with a model of DL-UVB-UV315-40W at 10-15°C, and the illumination time is 700 min, and solution B is obtained after the illumination ends; The solvent was distilled off under reduced pressure from solution B, and 0.25 g of photosterol L2 was obtained by preparative column separation. In this example, the yield of photosterol L2 was 25%.
Embodiment 2
[0044]Mix 1 g of ergosterol and 20 mL of tetrahydrofuran evenly to obtain solution A; at 10-15 ° C, first use LED ultraviolet lamp with model DL-UVC-UV254-30W to irradiate solution A, and the illumination time is 200min, and then use model DL- UVB-UV315-40W LED ultraviolet lamp irradiation, the irradiation time is 200min, and the solution B is obtained after the irradiation; the solvent B is distilled off under reduced pressure, and 0.25 g of photosterol L2 is obtained by preparative column separation. In this example, the yield of photosterol L2 was 25%.
Embodiment 3
[0046] Mix 1 g of 7-dehydropregnenolone acetate and 40 mL of tetrahydrofuran uniformly to obtain solution A; at 10-15 °C, irradiate solution A with an LED ultraviolet lamp with a model of DL-UVB-UV315-40W, and the illumination time is 750 min , solution B was obtained after irradiation; the solvent was distilled off under reduced pressure for solution B, and 0.22 g of pregnansterol acetate was obtained by preparative column separation. The yield of pregnansterol acetate in this example was 22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com