Method for synthesizing ursodeoxycholic acid by taking BA as raw material
A technology of ursodeoxycholic acid and raw materials, applied in the synthesis of ursodeoxycholic acid, in the field of synthetic ursodeoxycholic acid, can solve the problems of expensive raw materials, cumbersome steps, large pollution, etc., and achieve simple post-processing and low price , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
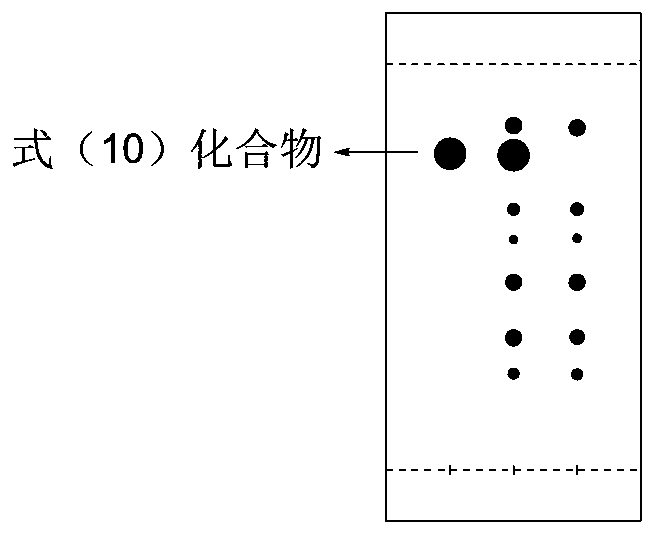
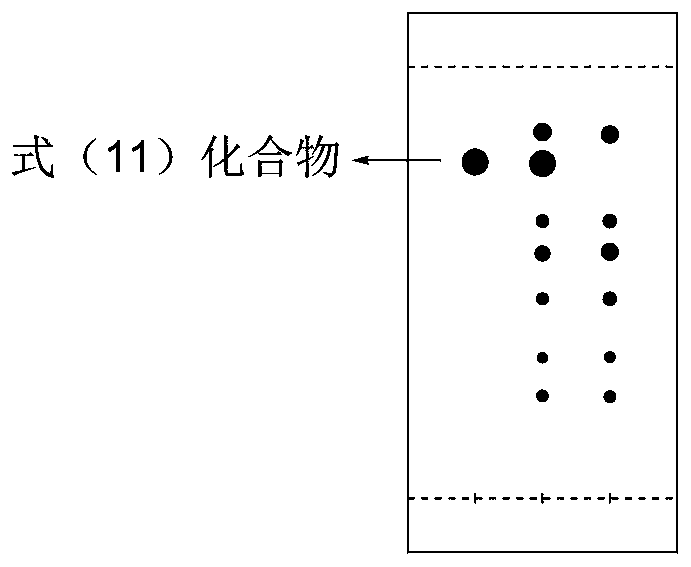
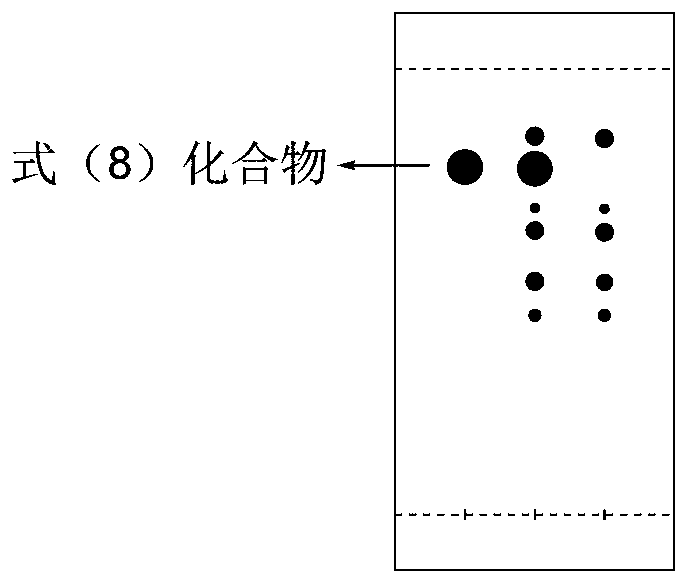
Image
Examples
Embodiment 1
[0117] The preparation of embodiment one formula (2) compound
[0118] Add BA (10.0g, 30.26mmol), p-toluenesulfonic acid (57mg, 0.30mmol), ethylene glycol (16.8mL, 302.60mmol), and triethyl orthoformate (15.1mL, 90.78mmol) in sequence in a 250mL single-necked flask And 150mL tetrahydrofuran, react at room temperature for 8h. After the reaction was completed, concentrate under reduced pressure, add 100 mL of water, extract with ethyl acetate (60 mL×3), wash with water (50 mL×2), wash with saturated sodium chloride solution (50 mL), dry over anhydrous sodium sulfate, concentrate under reduced pressure, and Purified by column chromatography (PE / EA=3 / 1, v / v), the compound of formula (2) (5.0 g, white solid) was obtained with a molar yield of 44%.
[0119] BA (10.0g, 30.26mmol), p-toluenesulfonic acid (57mg, 0.30mmol), ethylene glycol (16.8mL, 302.60mmol) and 300mL benzene were successively added into a 250mL single-necked flask, and the reaction was carried out under reflux and w...
Embodiment 2
[0121] The preparation of embodiment two formula (3) compound
[0122] The compound of formula (2) (5.0 g, 13.35 mmol), IBX (7.5 g, 26.70 mmol), 50 mL THF and 50 mL DMSO were successively added into a 250 mL one-necked flask, and reacted at room temperature for 5 h. After the reaction was detected by TLC, add water, filter with suction, extract with dichloromethane (50mL×3), wash with water (50mL×2), wash with saturated sodium bicarbonate solution (50mL), dry over anhydrous sodium sulfate, concentrate under reduced pressure, and Purified by column chromatography (PE / EA=3 / 1, v / v), the compound of formula (3) (4.9 g, white solid) was obtained with a molar yield of 98%.
[0123] Add formula (2) compound (10.1g, 26.96mmol), TEMPO (42mg, 0.27mmol), 100mL dichloromethane, sodium bicarbonate (3.1g, 36.40mmol) and tetrabutylammonium bromide successively in 500mL one-necked flask (870mg, 2.70mmol) in water (40mL), NCS (4.1g, 31.00mmol), react at 0°C for 5h. After the reaction was det...
Embodiment 3
[0125] The preparation of embodiment three formula (6) compound
[0126] The compound of formula (3) (1.0 g, 2.68 mmol), methoxyformylmethylene triphenylphosphine (1.7 g, 5.36 mmol) and 15 mL of toluene were successively added into a 100 mL one-necked flask, and the reaction was refluxed for 4 h. TLC detected that the reaction was complete, concentrated under reduced pressure, and purified by silica gel column chromatography (PE / EA=3 / 1, v / v) to obtain the compound of formula (6) (1.13 g, white solid), with a molar yield of 98%.
[0127] Sodium hydride (161mg, 4.02mmol) and 10mL tetrahydrofuran were added in a 100mL single-necked flask, and after stirring for 15min, trimethyl phosphonoacetate (0.65mL, 4.02mmol), compound of formula (3) (1.0g, 2.68mmol), 0 Reaction at ℃ for 4h. TLC detected that the reaction was complete, concentrated under reduced pressure, and purified by silica gel column chromatography (PE / EA=3 / 1, v / v) to obtain the compound of formula (6) (1.12 g, white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com