Cyp11b, cyp17, and/or cyp21 inhibitors
A technology of heteroaryl compounds, applied in the field of C-17-heteroaryl steroid compounds as CYP11B, CYP17 and/or CYP21 inhibitors, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
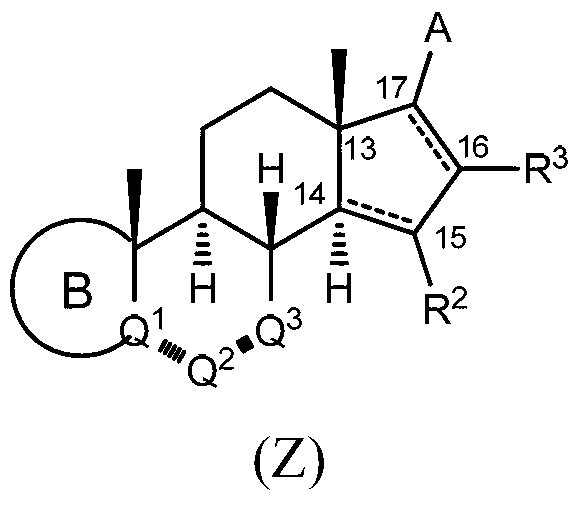
[0210] Example 1: In another example, compounds of formula (Z) having the structure of formula (I) are disclosed:
[0211]
[0212] in For -CH-CH=CH-, -C=CH-CH 2 -, -CH-CH 2 -CH 2 -, -CH-CH(OH)-CH 2 -, -CH-C(O)CH 2 -,or
[0213] each are independently single or double bonds;
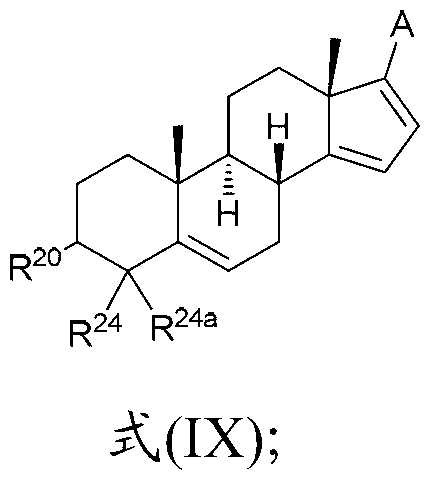
[0214] A is composed of 1, 2, 3 or 4 R as the case may be 4 Substituted heteroaryl;
[0215] Each R 4 When present, independently selected from halo, cyano, hydroxy, alkoxy, alkyl, alkenyl, haloalkyl, cycloalkyl, heterocycloalkyl, aryl, heteroaryl, C(O)R 13a , alkoxycarbonyl, -C(O)NR A R B , -NR 13 S(O) 2 R 13a , -NR 13a C(O)R 13a and -NR A R B composed of groups;
[0216] R A and R B independently selected from the group consisting of hydrogen, alkyl, haloalkyl, alkoxyalkyl, cycloalkyl, aryl, arylalkyl, heteroaryl and heteroarylalkyl; or
[0217] R A and R B Together with the nitrogen atom, a 4- to 7-membered heterocyclic ring having one or two heteroatoms is formed;
[02...
Embodiment 1a
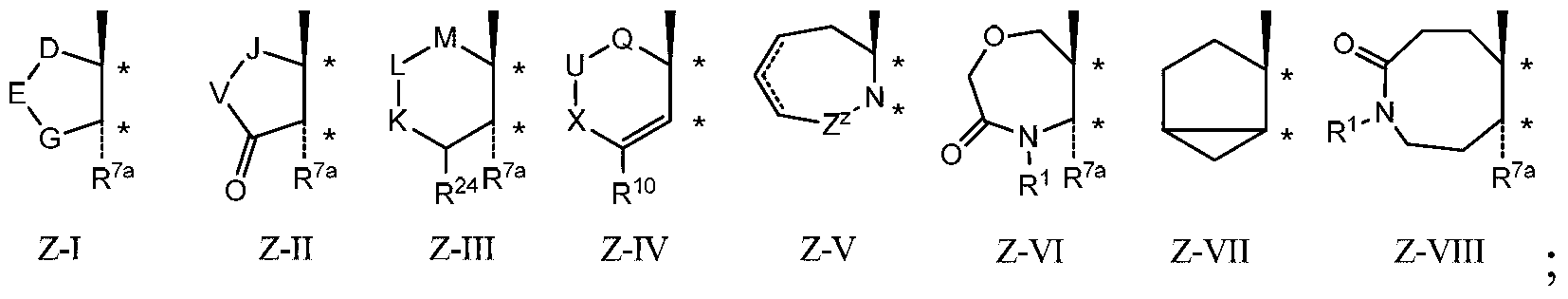
[0230] Example 1a: Compounds of Formula I conform to Formula IH
[0231]
[0232] in
[0233] Q 2 for CH 2 , CH(OH) or C(O);
[0234] D is (CH 2 ) d , where d is an integer from 1 to 3;
[0235] E for CH 2 、CR 14 R 14a , O, NR 1 , N-COR 1 , N-S(O) 0-2 (Alkyl) or N-COOR 1 ;
[0236] G is CH(CH 3 ), C(CH 3 ) 2 or (CH 2 ) e , where e is an integer from 1 to 3,
[0237] The restriction is that when E is CH 2 And when d+e is 3, then A is not unsubstituted furyl, unsubstituted thienyl, oxadiazolyl substituted by alkyl or phenyl or thiadiazolyl substituted by alkyl;
[0238] A is composed of 1, 2, 3 or 4 R as the case may be 4 Substituted heteroaryl;
[0239] Each R 1 independently selected from the group consisting of hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, alkoxyalkyl, hydroxy and haloalkoxyalkyl; wherein alkyl, cycloalkyl, alkenyl, alkynyl , alkoxyalkyl and haloalkoxyalkyl optionally consist of 1, 2 or 3 independently selected from halo, alkyl, al...
Embodiment 1f
[0255] Example 1f: Compounds of formula I conform to formula IJ
[0256]
[0257] in
[0258] D is (CH 2 ) d , where d is an integer from 1 to 3;
[0259] E for CH 2 、CR 14 R 14a , O, NR 1 , N-COR 1 , N-S(O) 0-2 (Alkyl) or N-COOR 1 ;
[0260] G is CH(CH 3 ), C(CH 3 ) 2 or (CH 2 ) e , where e is an integer from 1 to 3,
[0261] The restriction is that when E is CH 2 And when d+e is 3, then A is not unsubstituted furyl, unsubstituted thienyl, oxadiazolyl substituted by alkyl or phenyl or thiadiazolyl substituted by alkyl;
[0262] A is composed of 1, 2, 3 or 4 R as the case may be 4 Substituted heteroaryl;
[0263] Each R 1 independently selected from the group consisting of hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, alkoxyalkyl, hydroxy and haloalkoxyalkyl; wherein alkyl, cycloalkyl, alkenyl, alkynyl , alkoxyalkyl and haloalkoxyalkyl optionally consist of 1, 2 or 3 independently selected from halo, alkyl, alkenyl, aryl, heteroaryl, alkoxy, alkoxycarb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com