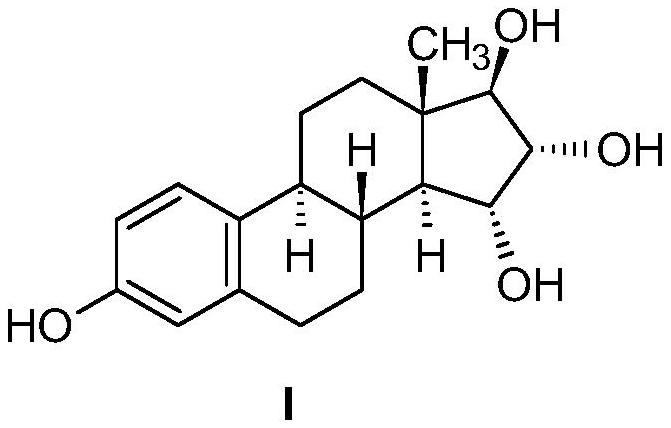
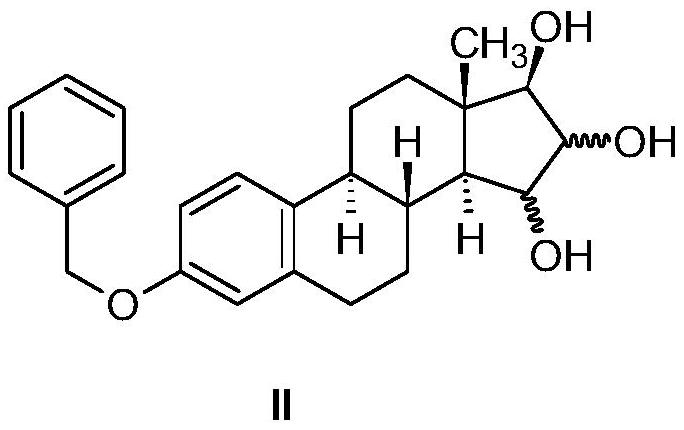
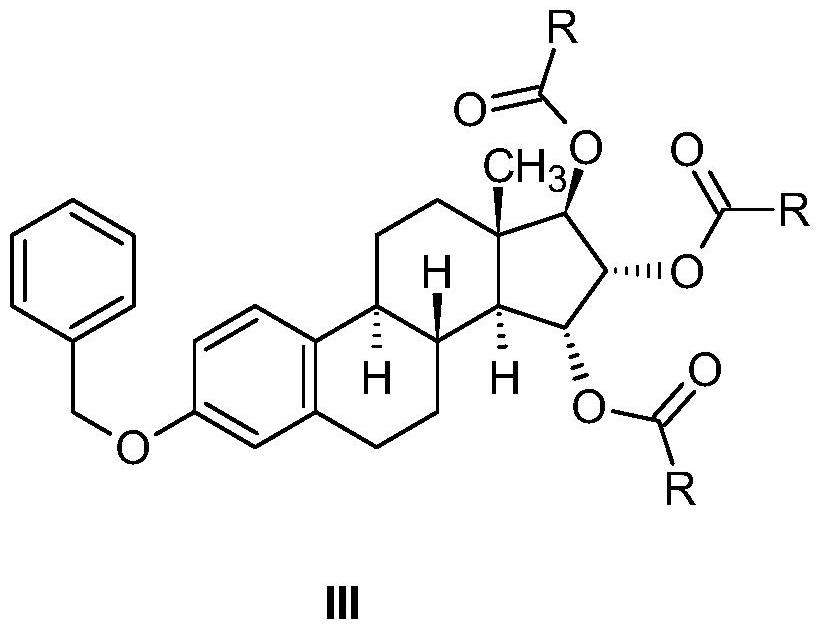
Industrial process for preparation of high purity estetrol
A technology for the use of estetrol, which is applied in the field of preparation of pharmaceutical compositions, general formulas and intermediates, and can solve problems such as low economy, unfavorable yield, and unresolved estetrol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] (15α,16α,17β)-3-(Benzyloxy)estra-1,3,5(10)-triene-15,16,17-triyltriacetate Method A (isolated)
[0100] a.) cis hydroxylation
[0101] (15α,16α,17β)-, and (15β,16β,17β)-3-(benzyloxy)estra-1,3,5(10)-triene-15,16,17- triol
[0102] At 20-25°C, in N 2 Under the atmosphere, 40 mg of potassium osmate dihydrate (K 2 OSo 4 2H 2 O) Suspended in 100 mL of 2-butanone (methyl ethyl ketone), 7.7 mL of purified water and 1.1 g of trimethylamine N-oxide dihydrate were added. 2.0 g (5.5 mmol) of 3-benzyloxy-estra-1,3,5(10),15-tetraen-17-ol (WO2004 / 041839 (Pantarhei), Example 7) was dissolved in 40 mL of 2 -butanone and added dropwise to the reaction mixture. Then, at N 2 The reaction mixture was stirred at 20-25°C for 28 hours under atmosphere. The reaction was monitored by TPLC (n-heptane:acetone 1:1).
[0103] Post-processing: 25 mL of 10% Na 2 S 2 o 5 The solution was added to the mixture, followed by the addition of 100 mg of activated carbon, followed by stirr...
Embodiment 2
[0128] (15α,16α,17β)-3-Hydroxyestro-1,3,5(10)-triene-15,16,17-triyltriacetate
[0129] Method A
[0130] At 20-25°C, in N 2 Under atmosphere, 25.7g (49.36mmol) of (15α,16α,17β)-3-(benzyloxy)estra-1,3,5(10)-triene-15,16,17-triyltriethyl The acid ester (Example 1) was dissolved in 315 mL of ethyl acetate. 770 mg of 10% palladium on carbon catalyst was suspended in 19 mL of cryogenic ethyl acetate and then added to the solution. Will N 2 Atmosphere changed to H 2 atmosphere, and the reaction mixture was stirred at 20-25 °C for 3 hours at atmospheric pressure.
[0131] Post-processing : The catalyst was filtered off, washed with ethyl acetate, and concentrated to a final volume under reduced pressure, then n-heptane was added, and the suspension was kept at 0-5 °C for 1 hour, then filtered, and used on the filter with The crystalline product was washed with n-heptane and dried in vacuum at 40 °C to constant weight. Thus, 19.88 g (93.55%) of white crystalline product were ...
Embodiment 3
[0142] (15α,16α,17β)-3-(Benzyloxy)estra-1,3,5(10)-triene-15,16,17-triyltricarboxylate
[0143] 5.00 g of (15α,16α,17β)-3-(benzyloxy)estra-1,3,5(10)-triene-15,16,17-triol (Example 1, method "A" , step a)) was dissolved in 73 mL of pyridine and cooled to 0° C., then between about 0-10° C., in about 25 minutes, 49 mL of formic acid cooled to 0° C. and 18.3 mL of A mixture of mixed anhydrides made from the anhydride of acetic acid. After stirring for 1 hour, 305 mL of water was added to the reaction mixture, and the resulting white precipitate was filtered off and washed with water. The dried crude product weighed 5.65 g (93.23%).
[0144] The crude product - according to method B step c) of example 1 - was recrystallized from methanol to afford 3.92 g (69.4%) of the pure title product as white crystals.
[0145] Purity (HPLC): 99.2% ααβ-isomer, 0.05% βββ-isomer (area).
[0146] Mp.:153.5-154.3℃
[0147] EI HRMS: M=478.19866; δ=0.06ppm; C 28 h 30 o 7
[0148] 1 H NMR (49...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com