Orodispersible dosage unit containing an estetrol component
a technology of estetrol and dosage unit, which is applied in the direction of organic active ingredients, drug compositions, sexual disorders, etc., can solve the problem provide administration, and achieve the effect of rapid onset of action, easy manufacturing, and avoidance of first-pass liver exposur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0137]A sublingual tablet is prepared by means of the procedure described below.
[0138]A tabletting mixture having the composition shown in Table 1 is prepared by dry blending, using a low shear mixer.
TABLE 1IngredientsWt. %Milled estetrol112.5Mannitol47.5Lactose30PVP (polyvinylpyrrolidone)4Sodium crosscarmellose4Flavour0.5Aspartame1Magnesium stearate0.51D(v;0.5) = 15 μM
[0139]The tabletting mixture is compressed into 80 mg round tablets with a diameter of 6.5 mm. The estetrol content of these tablets is 10 mg.
example 2
[0140]A sublingual tablet is prepared by means of the procedure described below.
[0141]A tabletting mixture having the composition shown in Table 2 is prepared by dry blending using a low shear mixer.
TABLE 2IngredientsWt. %Milled estetrol112.5Mannitol37.5Xylitol10Microcrystalline cellulose33Sodium starch glycolate5Flavour0.5Aspartame1Magnesium stearate0.51D(v;0.5) = 15 μm
[0142]The tabletting mixture is compressed into 80 mg round tablets with a diameter of 6.5 mm. The estetrol content of these tablets is 10 mg.
example 3
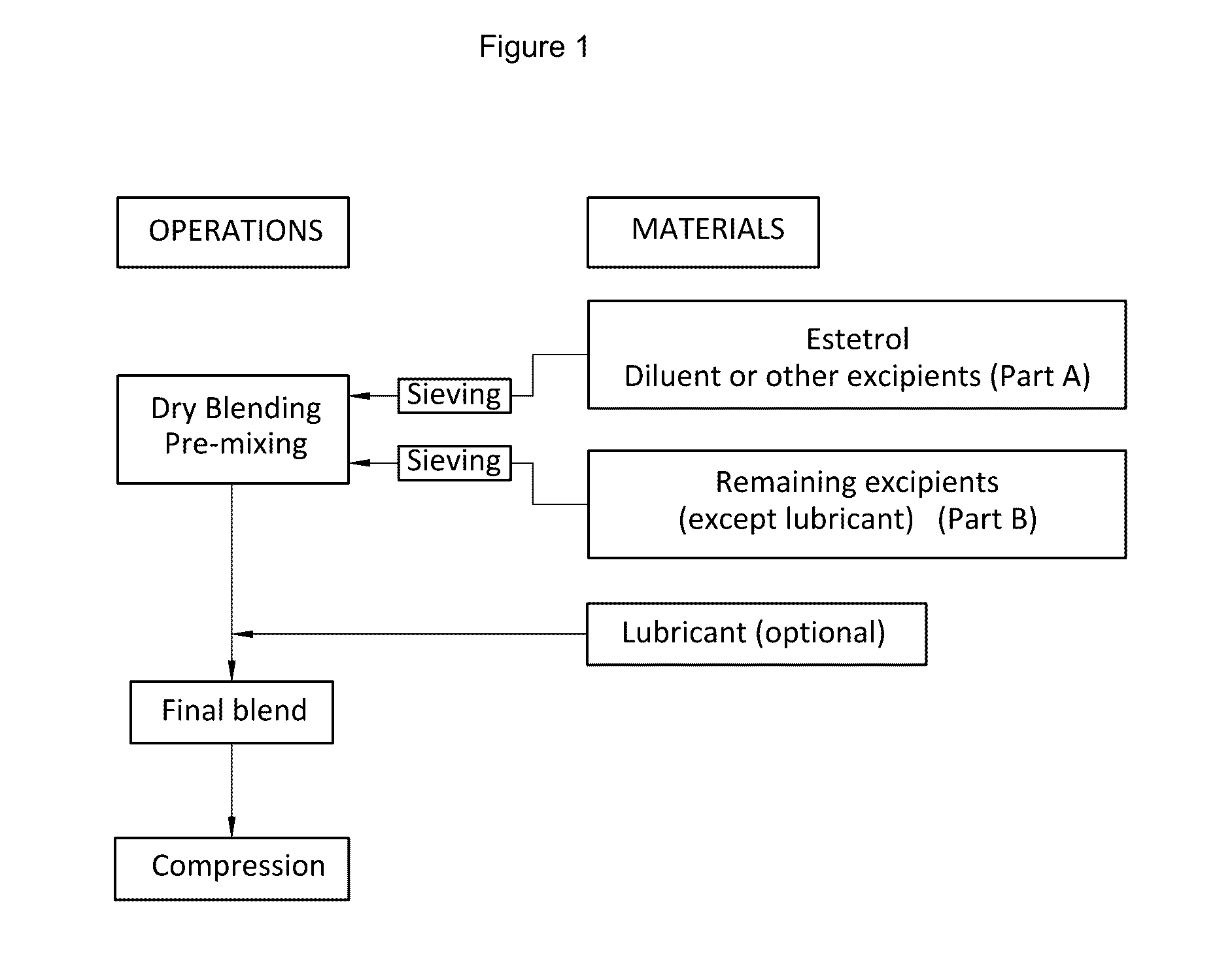
[0143]Five different sets of sublingual tablets (formulations A to E) were prepared by means of the procedure described below and illustrated in FIG. 1.
[0144]The target amounts of estetrol per tablet were as follows: 100 μg for formulation A, 1 mg for formulation B, and 10 mg for formulations C, D and E.
[0145]The target weights for the tablets were as follows: 30 mg for formulation A, 1000 mg for formulation B, and 80 mg for formulations C, D and E.
[0146]The estetrol was mixed with a part of the main diluent and screened over a 800 μm screen. All other excipients were also screened over a 800 μm screen.
[0147]The materials were weighed and transferred into the mixing container (except for magnesium stearate) and mixed for 15 minutes. Finally, magnesium stearate was added and mixed for a further 3 minutes.
[0148]Compression was executed using a single punch machine equipped with a proper punch (5 mm punch for 30 mg tablets (A), 6 mm for 80 mg tablets (C, D and E) and 15 mm for 1000 mg ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com