Treatment of advanced estrogen receptor positive breast cancer
An estrogen receptor, estrogen technology, applied in the field of breast cancer treatment, can solve problems such as harmful side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
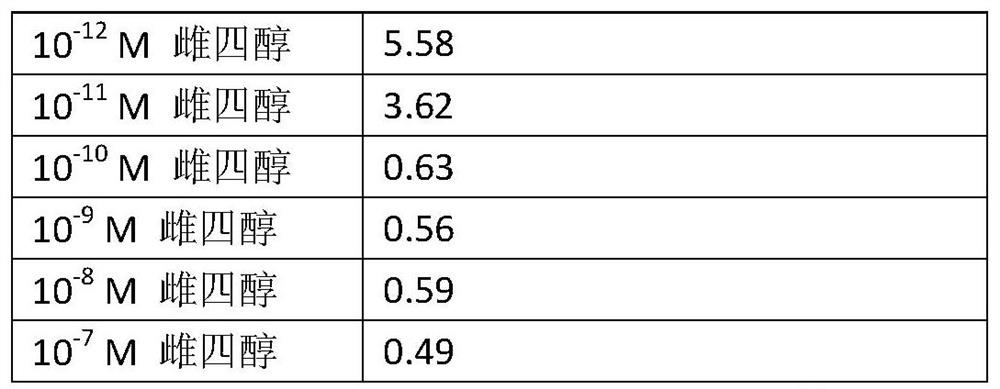
[0077] To assess the potential of the estetrol component to inhibit the growth of estrogen-deprived breast cancer cells, an in vitro study of long-term estrogen-deprived MCF7 breast cancer cells (LTED cells) was performed. LTED cells were deprived of estrogen, making them a suitable in vitro model to study drug effects in women on long-term use of inhibitors of estrogen activity.
[0078] LTED cells were plated in 6-well plates at a density of 30,000 cells per well. Cells were maintained in phenol red-free IMEM with 5% charcoal-treated FBS (DCC-FBS). On day 3, replace the medium with fresh phenol red-free IMEM with 5% DCC-FBS. Cells were then exposed to 6 different concentrations of estetrol ranging from 10 -12 M to 10 -5 M, or exposure to ethanol as vehicle control. For all experiments, the final vehicle concentration was 0.1% ethanol. Each treatment was performed in duplicate.
[0079] On day 5, the medium was changed and on day 7 the plates were analyzed for cytometry...
Embodiment 2
[0086] Plasma levels of estetrol were tested on days 7 and 14 in 9-10 female patients for each group taking (orally administered) 2, 10, 20 and 40 mg estetrol per day. For each dose group, the average trough level for all patients on these two days was calculated.
[0087] As shown in Table 2, the results showed good dose linearity. Furthermore, this study shows that these high dose levels of estetrol are well tolerated, whereas comparable doses of other estrogens can cause significant side effects, such as nausea, that negatively impact QOL.
[0088] Table 2 - Trough and C of estetrol as a function of oral dose administered max plasma level
[0089] Estimated oral dose per day (mg) Trough plasma level (pg / mL) C max Plasma level (pg / mL)
Embodiment 3
[0091] A multicentre study was conducted with recruitment lasting approximately 9 months.
[0092] Conduct an open-label, phase I / IIa trial, dose-escalation study with a 3+3 cohort design to determine the recommended dose of E4 for the treatment of patients with advanced breast cancer. Following completion of the Phase I portion of the study (ie, 4 weeks of treatment), patients received a further 8 weeks of treatment at their respective Phase I dose levels (Phase IIa portion of the study). Patients were treated for 12 weeks. Treatment continued after 12 weeks of treatment until patients withdrew from the study due to unacceptable adverse events (AEs) or disease progression. These patients were followed according to clinical practice including radiation oncology assessment.
[0093] target group : A total of at least 9 patients with ER+ locally advanced and / or metastatic breast cancer who had exhausted all treatment options were included in the study. Patients who have bee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com