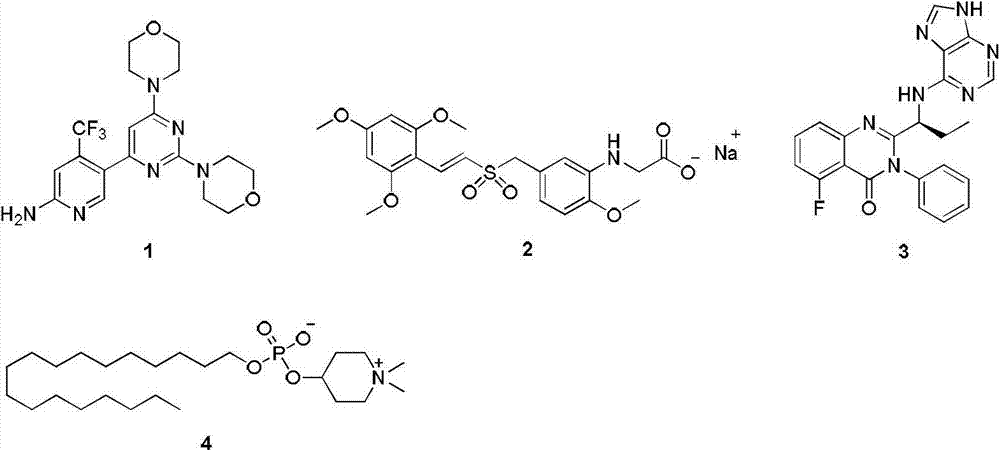
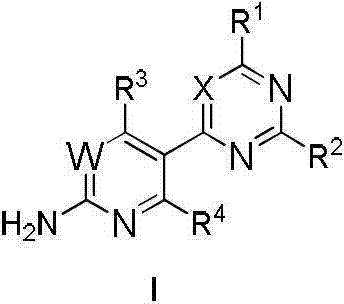
Phosphoinositide 3-kinase (P13K) inhibitor, pharmaceutical composition containing P13K inhibitor, and application of phosphoinositide kinase inhibitor and pharmaceutical composition
A kind of kinase inhibitor, hydrate technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
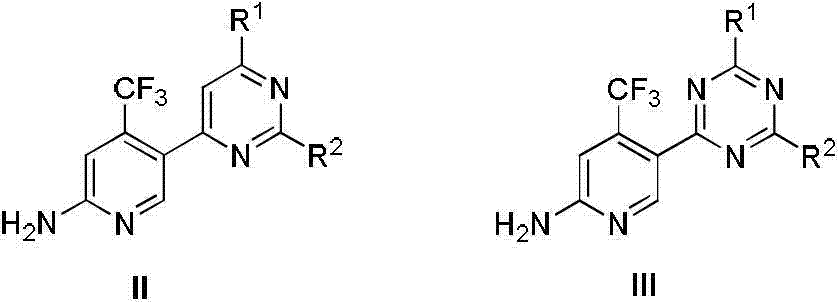
[0130] Example 1: Synthesis of pyrimidine compound ZJQ-04.
[0131] Step 1: Synthesis of 5-bromo-4-(trifluoromethyl)pyridin-2-amine.
[0132] The structural formula of 5-bromo-4-(trifluoromethyl)pyridin-2-amine:
[0133] Synthetic method: Dissolve 4-trifluoromethyl-2-aminopyridine (10 g, 61.69 mmol) in CH 2 Cl 2 (100 mL), bromosuccinimide (NBS, 12.08 g, 67.86 mmol) was added in portions at room temperature, and reacted overnight at room temperature in the dark. The reaction system uses CH 2 Cl 2 (100mL) diluted with saturated NaHSO 3 Wash twice, wash once with saturated NaCl aqueous solution, and dry over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was separated and purified by column chromatography, eluent: petroleum ether / ethyl acetate=4 / 1, to obtain 13.08 g of the target product as a brown solid, yield: 87.96%.
[0134] The NMR data of the product are 1 H NMR (400MHz, CDCl 3 )δ: 8.28(s, 1H), 6.78(s, 1H), 4.82(s, 2H...
Embodiment 2
[0170] Example 2: Synthesis of pyrimidine compound ZJQ-05.
[0171] Step 1: (2R,4R)-1-tert-butyl-2-methyl-4-((tert-butyldimethylsilyl)oxy)tetrahydropyrrole-1,2-dicarboxylate synthesis.
[0172] The structural formula of (2R,4R)-1-tert-butyl-2-methyl-4-((tert-butyldimethylsilyl)oxy)tetrahydropyrrole-1,2-dicarboxylate:
[0173] Synthetic method: Dissolve (2R,4R)-1-tert-butyl-2-methyl 4-hydroxytetrahydropyrrole-1,2-dicarbonate (5g, 20.40mmol) in dichloromethane (40mL), DIPEA (7.46mL, 44.87mmol) was cooled to -40°C, tert-butyldimethylsilyl triflate (5.63mL, 24.48mmol) was added dropwise and stirring was continued for 3h. A large amount of water was added to quench the reaction, and dichloromethane was added for extraction, dried over anhydrous sodium sulfate, concentrated, and purified by column, eluent: PE / EA=3 / 1, and 6.63 g of the target product was obtained with a yield of 90.45%.
[0174] The mass spectrum data of the product is LC-MS: 360.2 (M+H). Same as in WO 20091371...
Embodiment 3
[0200] Example 3: Synthesis of pyrimidine compound ZJQ-22.
[0201] (-)-5-(6-morpholine-2-((4RS, 7RS)-tetrahydro)-2H-[1,4]dioxin)[2,3-c]pyrrol-6(3H)-yl )pyrimidin-4-yl)-4-(trifluoromethyl)pyridin-2-amine, the structural formula of compound ZJQ-22:
[0202]
[0203] Synthesis method: ZJQ-04 prepared in Example 1 is resolved, separation conditions: reversed-phase chiral preparative column (lu 5u cellose-2 of Pheromones), mobile phase: methanol / water=95 / 5; purity: 99.90%; retention time: 13.94min, the resolution product ZJQ-22 was obtained.
[0204] The high-resolution mass spectrometry data of the resolved product is HRMS (ESI): m / z[M+H] + calcd.for[C 20 h 24 f 3 N 6 o 3 ] + : 453.1856, found: 453.1845. Optical rotation data is From the above data, it can be seen that the product prepared by the above method is the target product (-)-5-(6-morpholine-2-((4RS, 7RS)-tetrahydro)-2H-[1,4]dioxin) [2,3-c]pyrrol-6(3H)-yl)pyrimidin-4-yl)-4-(trifluoromethyl)pyridin-2-amine,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com