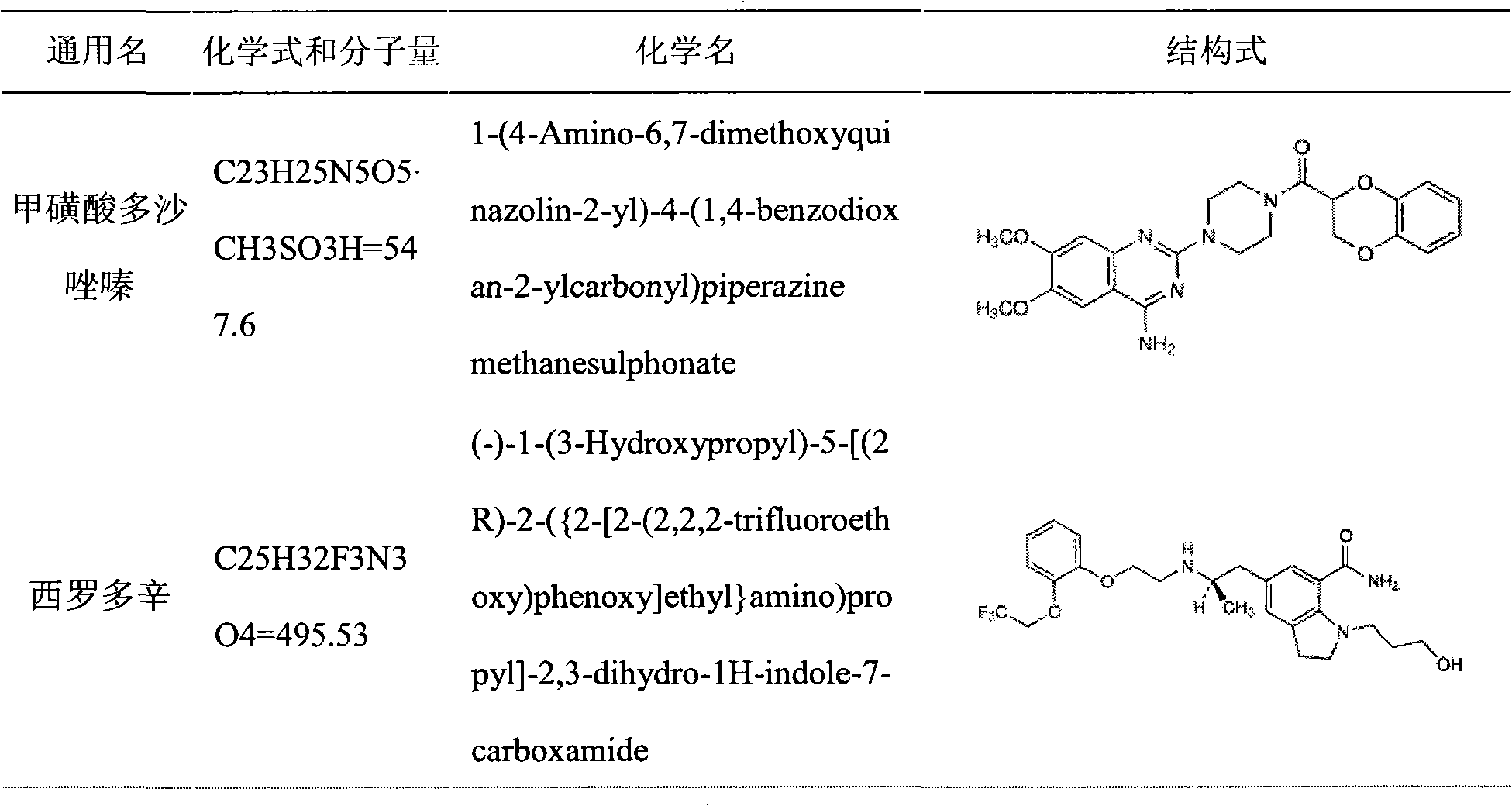
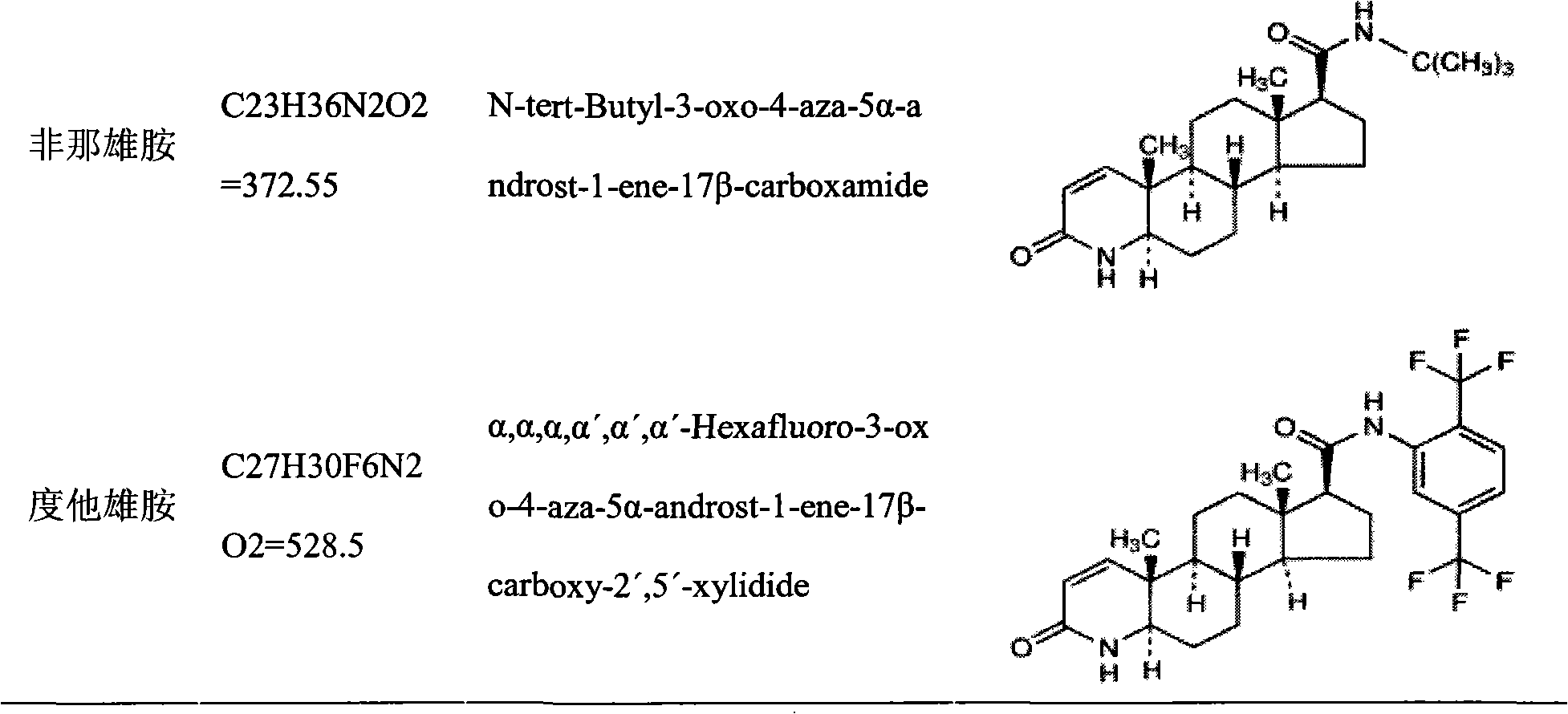
Medicinal composition containing insoluble medicament
A composition and drug technology, applied in the field of pharmaceutical preparations, to achieve the effects of high dispersion, good stability, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
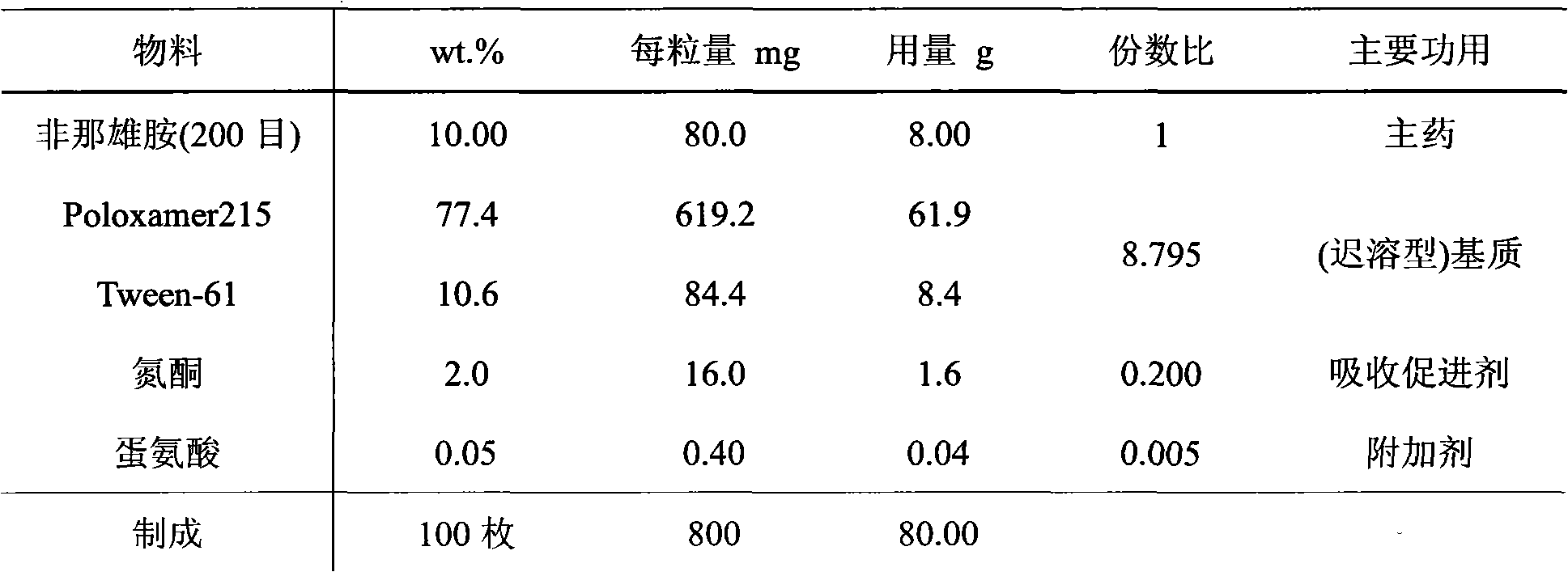
[0045] The preparation of embodiment 1 suppository A
[0046] 1. Prescription A (quantity of 100 suppositories)
[0047]
[0048] 2. The preparation method of suppository A
[0049] 1) Take the main ingredients in the prescription and pass through a 200-mesh sieve, and pass through a 100-mesh sieve for other solid materials unless otherwise specified;
[0050] 2) The matrix is melted, filtered, and sterilized at 45-80°C; the main drug in the prescribed amount is miscible with the enhancer (auxiliary, latent) solvent and / or absorption enhancer or is properly heated and melted to form a liquid dispersion;
[0051] 3) Weigh the above-mentioned melted matrix of the prescribed amount, keep warm at 45-55°C, add other excipients of the prescribed amount under stirring and mix evenly, continue to add the above-mentioned liquid dispersion containing the main drug under stirring, and fully mix under the condition of heat preservation. Homogenize to obtain the whole material mixtu...
Embodiment 2
[0054] The preparation of embodiment 2 suppositories B
[0055] 1. Prescription B (amount of 100 suppositories)
[0056]
[0057]
[0058] 2. Preparation method of suppository B
[0059] With embodiment 1.
Embodiment 3
[0060] The preparation of embodiment 3 suppositories C
[0061] 1. Prescription C (amount of 100 suppositories)
[0062]
[0063] 2. The preparation method of suppository C
[0064] With embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com