High water-soluble prodrug, and its preparing method and pharmaceutical use
A prodrug and water-soluble technology, applied in the field of prodrugs, can solve the problems of decreased water solubility and unsuitability for clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
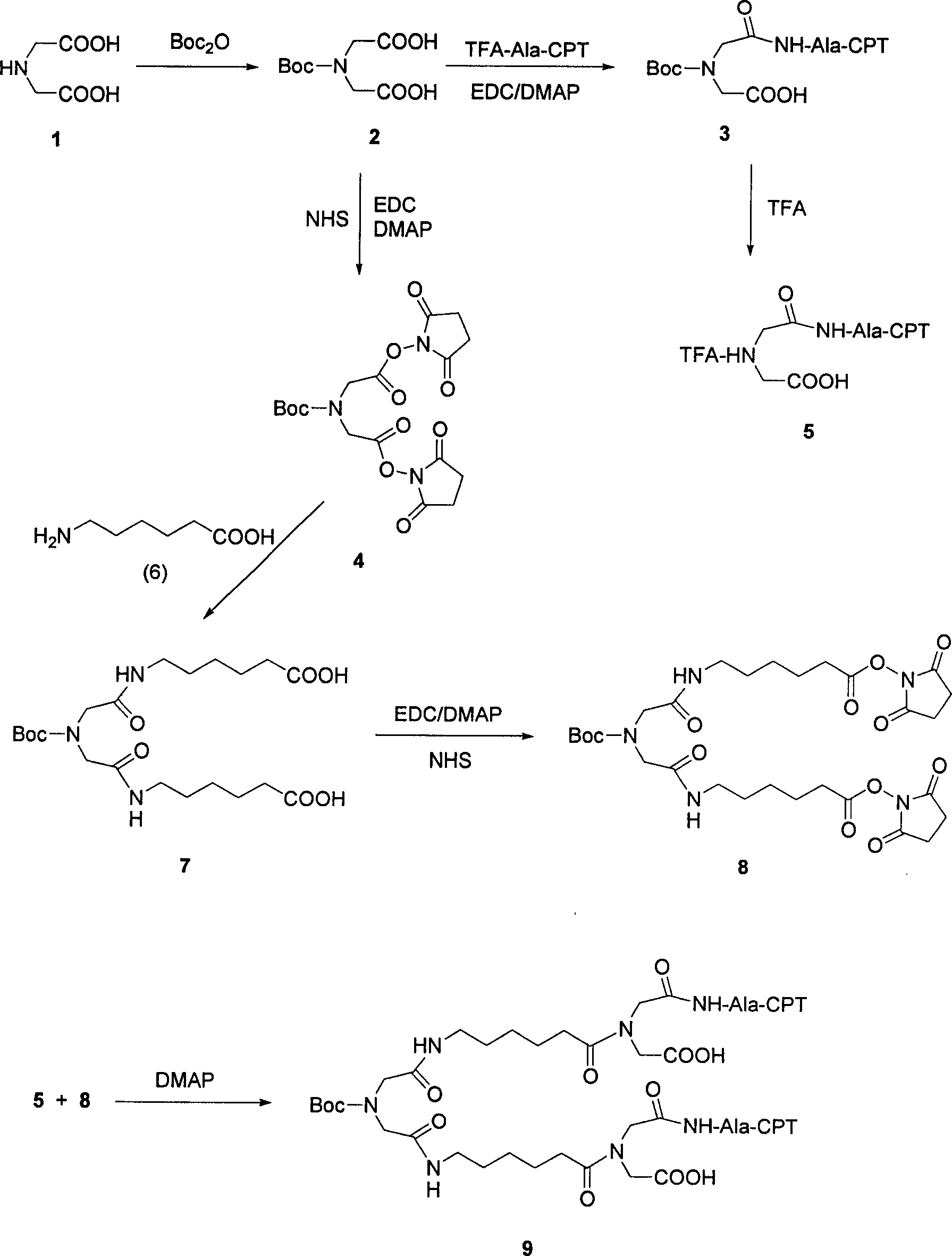
[0152] Iminodiacetic acid (compound 1, 5 g, 37.57 mmol) was dissolved in dichloromethane solution (DCM, 100 mL), diisobutyl dicarbonate (9.02 g, 41.32 mmol) was added and the mixture was stirred at room temperature for 2 hours react. Afterwards, the mixture was extracted with 0.1N HCl (3×100 mL), and the organic layer was washed with magnesium sulfate (MgSO 4 ), filtered and evaporated to give compound 2 (7.88 g, 33.81 mmol, 90% yield). 1 H NMR (300MHz, CDCl 3 )δ11.0(s, 2H), 3.90(s, 4H), 1.40(s, 9H); 13 C NMR (75.4MHz, CDCl 3 ) δ 176.0, 156.3, 70.9, 54.2, 28.7.
Embodiment 2
[0154] In the solution of compound 2 (3.94g, 16.91mmol), add the solution containing 4-dimethylaminopyridine (DMAP, 4.13g, 33.81mmol), 20(S)-camptothecin-(S) A DCM solution of alanine TFA salt (8.76g, 16.91mmol) and 1-(3-dimethylaminopropane)-3-acetaldehyde carbodiimide hydroxide (EDC, 4.86g, 25.36mmol) ( 400mL). The mixture was stirred and warmed to room temperature for two hours. with 0.1M NaHCO 3 (3×200mL), 0.1N HCl (3×200mL) and water (200mL) washed the reactant, and the organic layer was washed with MgSO 4 Drying, filtration and evaporation afforded compound 3 (9.42 g, 15.21 mmol, 90%). 1 H NMR (300MHz, CDCl 3 )δ11.0(s, 1H), 8.06(d, 1H), 8.0(d, 1H), 7.65(m, 2H), 7.57(d, 1H), 7.42(d, 2H), 6.74(s, 1H ), 4.75(s, 2H), 4.60(m, 1H), 3.90(s, 2H), 3.85(s, 2H), 1.96(q, 2H), 1.40(s, 9H), 0.96(t, 3H) ; 13 C NMR (75.4MHz, CDCl 3 )δ 176.0, 172.0, 170.9, 160.9, 160.7, 156.3, 149.2, 145.9, 140.3, 134.5, 129.2, 128.7, 128.3, 127.3, 127.2, 126.6, 125.9, 105.8, 91.7, 70.9, 66.2, 5 ...
Embodiment 3
[0156] In the aqueous solution of compound 2 (3.94g, 16.91mmol), add cooling to 0 ℃ containing 4-dimethylaminopyridine (DMAP, 4.13g, 33.81mmol), and M hydroxysuccinimide (NHS, 3.89g , 33.82 mmol) and 1-(3-dimethylaminopropane)-3-e acetaldehyde carbodiimide hydroxide (EDC, 6.48 g, 33.82 mmol) in DCM (400 mL). The mixture was stirred and warmed to room temperature for two hours. The reaction was washed with 0.1N HCl (3×200 mL), and the organic layer was dried under reduced pressure (using MgSO 4 ), filtered and evaporated to give compound 4 (6.86 g, 16.06 mmol, 90%). 1 H NMR (300MHz, CDCl 3 )δ3.90(s, 4H), 2.73(t, 8H), 1.40(s, 9H); 13 C NMR (75.4MHz, CDCl 3 )δ 176.0, 168.5, 156.3, 70.9, 49.1, 28.7, 22.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com