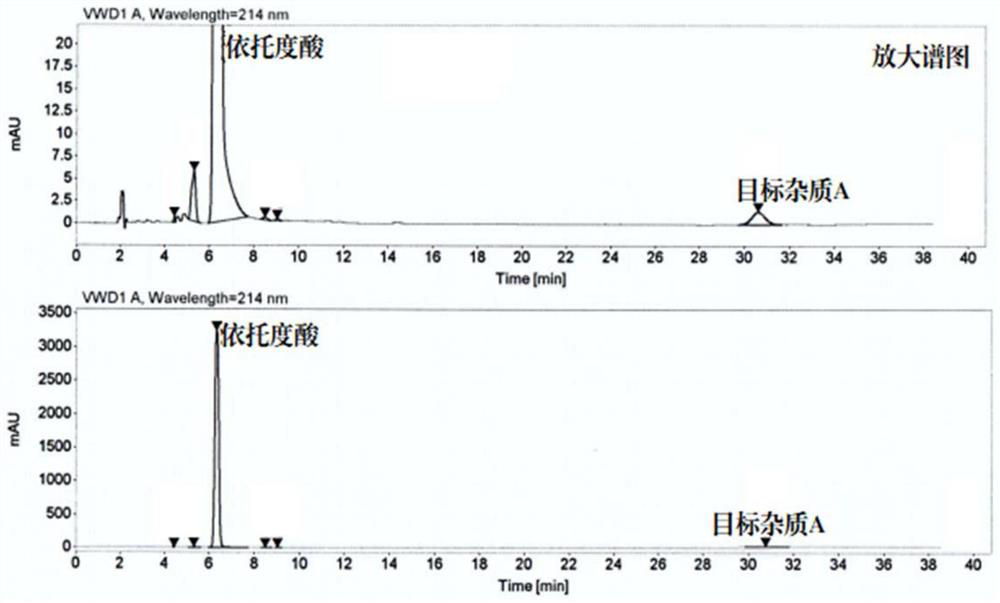
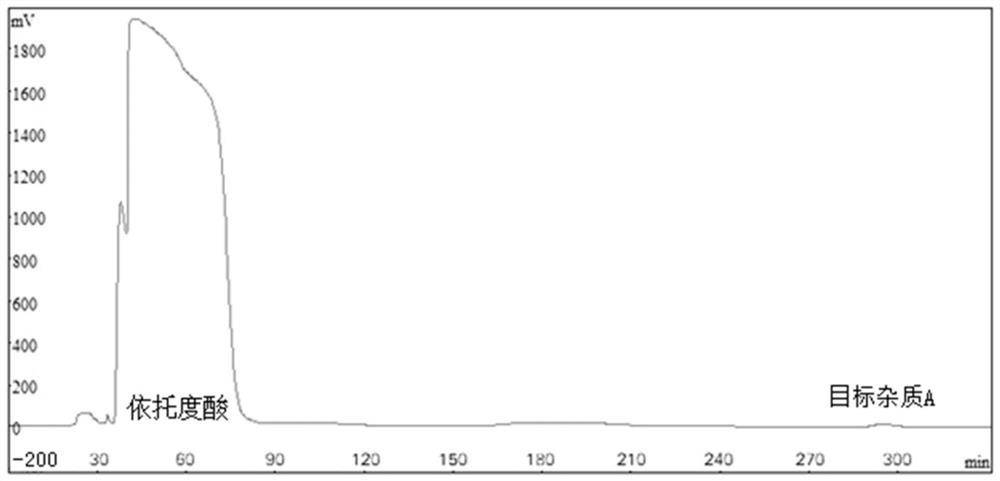
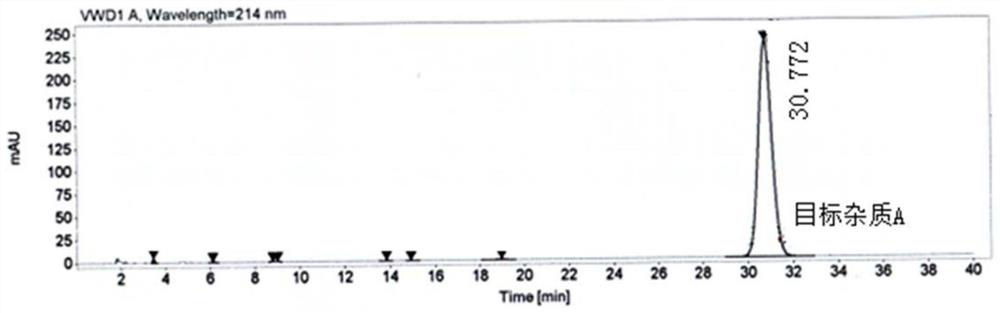
Method for separating and preparing trace impurity in etodolac bulk drug
A technology of etodolac and trace impurities, applied in the direction of organic chemistry, can solve the problems of high cost, difficult separation, long separation and purification time, etc., and achieve the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, a kind of method that separates and prepares impurity A from etodolac bulk drug, separates impurity A from etodolac bulk drug with countercurrent chromatograph, carries out the following steps successively:
[0047] 1), preparation of two-phase solvent:
[0048] Solvent system ①: the volume ratio of normal hexane: ethyl acetate: ethanol: water = 3:2:3:2 is poured into the liquid separator after preparation to form two kinds of mutually immiscible two-phase solvents, respectively The upper and lower phases of the solvent system ①;
[0049] Solvent system ②: the volume ratio of normal hexane: ethyl acetate: ethanol: water = 2:1:2:1 is poured into the liquid separator after preparation to form two kinds of two-phase solvents that are mutually immiscible, and respectively obtain The upper and lower phases of the solvent system ②;
[0050] 2), 4ml solvent system ① upper phase and 4ml solvent system ① lower phase are mixed, get mixed solvent ①; 500mg etodolac ...
Embodiment 2-1
[0069] Embodiment 2-1, a method for separating and preparing impurity A from etodolac bulk drug:
[0070] Change the injection flow rate of the mobile phase from 5.0ml / min to 6.0ml / min in step 2) of Example 1, and the rest are the same as in Example 1. The finally obtained impurity A has a purity of 96.4% and a recovery rate of 87.7%.
Embodiment 2-2
[0071] Embodiment 2-2, a method for separating and preparing impurity A from etodolac bulk drug:
[0072] Change the injection flow rate of the mobile phase from 5.0ml / min to 4.0ml / min in step 2) of Example 1, and the rest are the same as in Example 1. The final obtained impurity A has a purity of 96.2% and a recovery rate of 85.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com