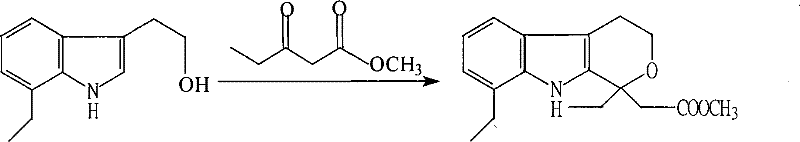
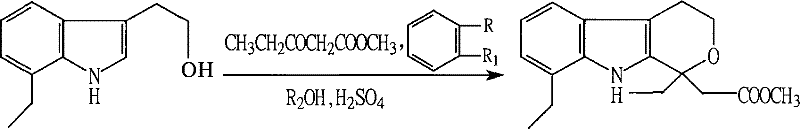Method for preparing etodolac methyl ester
A technology of methyl etodolac and methyl oxopentanoate, which is applied in the field of preparing indole non-steroidal anti-inflammatory analgesic etodolac, which can solve the problems of unsuitable industrialized production, many post-processing wastes, and high production costs. problems, to achieve the effect of low production cost, less three wastes and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step A: Add 70.0 grams (content 79.3% W / W, 0.2935 moles) of 7-ethyl tryptophanol, 120 milliliters of anhydrous methanol, 360 milliliters of benzene and 45.8 grams of Methyl 3-oxopentanoate was cooled to 0~-5°C with stirring, and 86 g of concentrated sulfuric acid was added dropwise, and the mixture was kept at 0~-5°C and stirred for 1 hour.
[0017] Step B: After the reaction is completed, the layers are statically separated, the acid layer is separated, the acid phase is extracted once with toluene, the organic phases are combined, and neutralized with an aqueous solution of sodium carbonate. The organic phase was distilled to dryness under reduced pressure. Recrystallization with methanol gave 88 g (yield 99.55%) of methyl etodolac. Mp=129.5~131℃
Embodiment 2
[0019] Step A: Add 70.0 grams (content 79.3% W / W, 0.2935 moles) of 7-ethyl tryptophanol, 120 milliliters of anhydrous methanol, 480 milliliters of toluene and 45.8 grams of Methyl 3-oxopentanoate was cooled to 0~-5°C with stirring, and 86 g of concentrated sulfuric acid was added dropwise, and the mixture was kept at 0~-5°C and stirred for 1 hour.
[0020] Step B: Same as Step B of Example 1.
Embodiment 3
[0022] Step A: add 70.0 grams (content 79.3% W / W, 0.2935 mole) 7-ethyl tryptophanol, 120 milliliters of anhydrous methanol, 600 milliliters of dimethylbenzene and 45.8 milliliters in the 1000ml four-necked reaction flask that stirrer, thermometer are equipped with gram of 3-oxopentanoic acid methyl ester, stirred and lowered to 0~-5°C, added dropwise 86 grams of concentrated sulfuric acid, kept at 0~10°C and stirred for 1 hour.
[0023] Step B: Same as Step B of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com