Etodolac ionic salt as well as preparation method and application thereof
A technology of etodolac and ionic salts, applied in the field of pharmaceutical and cosmetic compounds, can solve the problems of staying in the application range, limiting the application effect and application prospect, etc., and achieves the effects of improving absorption, good safety and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
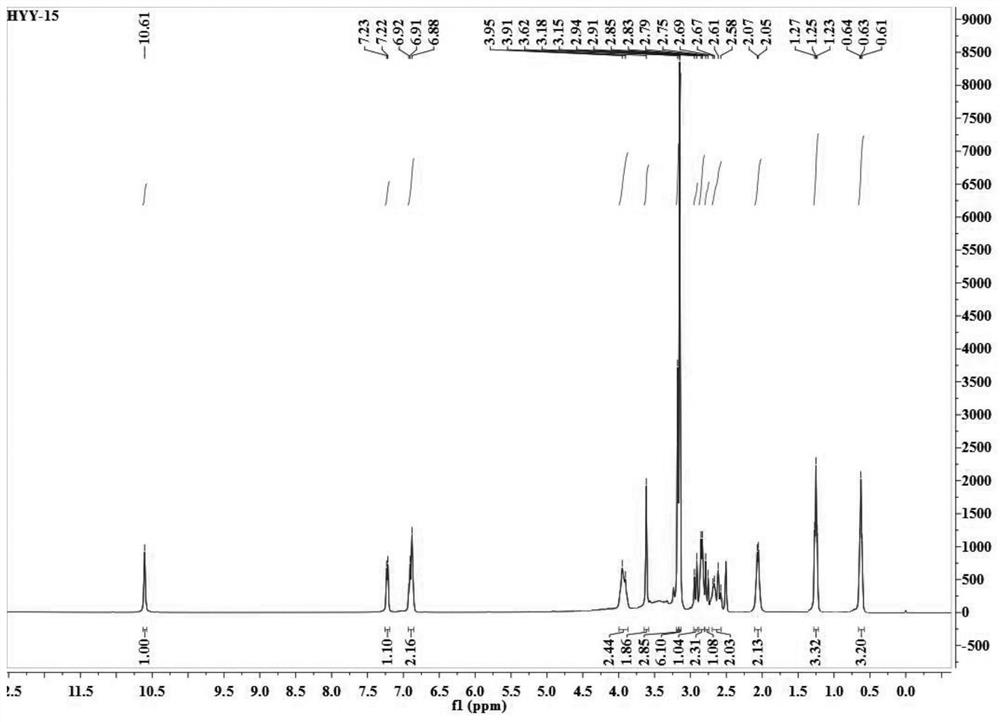
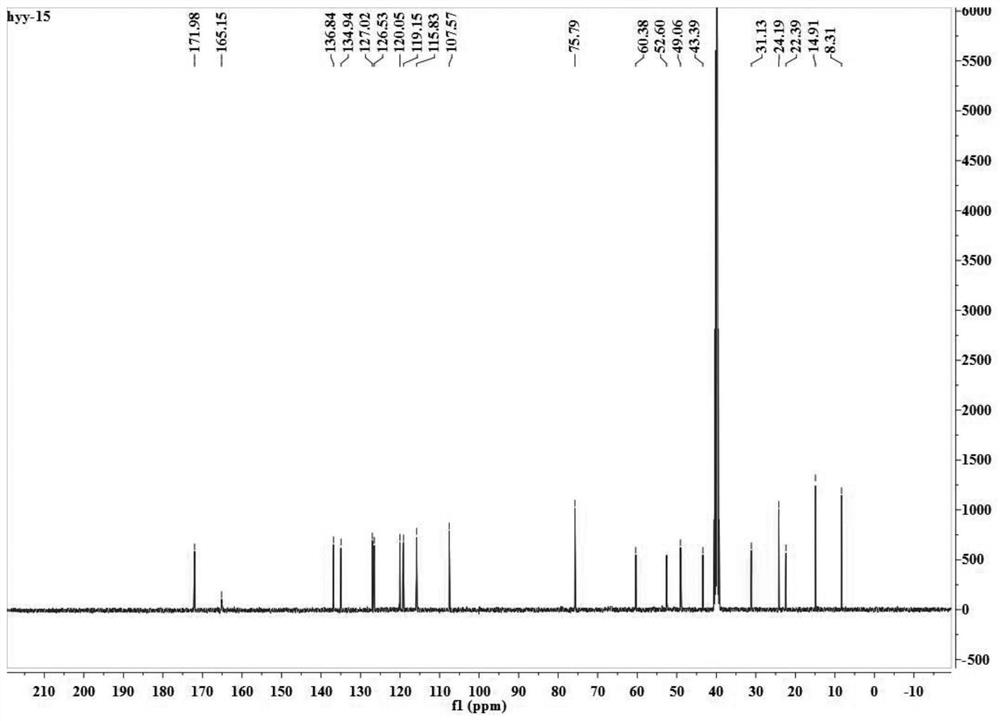
[0040] The present embodiment provides a kind of etodolac ion salt, specifically etodolac betaine ion salt, which is prepared through the following steps:
[0041] (1) Under argon atmosphere, dissolve 3mmol etodolac in the reactor with 5mL of ethanol, dissolve 3mmol betaine with 5mL of ethanol, add the ethanol solution of betaine dropwise into the reactor that is dissolved with etodolac , heated to 60°C, ionized modification reaction for 24 hours;
[0042] (2) after completion of the reaction, concentrate the solution to 1 / 8 of the reaction solution under vacuum conditions, freeze and crystallize, and obtain the product of etodolac betaine ionic salt through filtration, washing and separation, dry in a vacuum oven for 48 hours, Obtain pure etodolac betaine ionic salt.
[0043] figure 1 and figure 2 Respectively represent the proton nuclear magnetic resonance spectrum and the carbon spectrum of the etodolac betaine ion salt, and the specific nuclear magnetic data are: 1 H ...
Embodiment 2
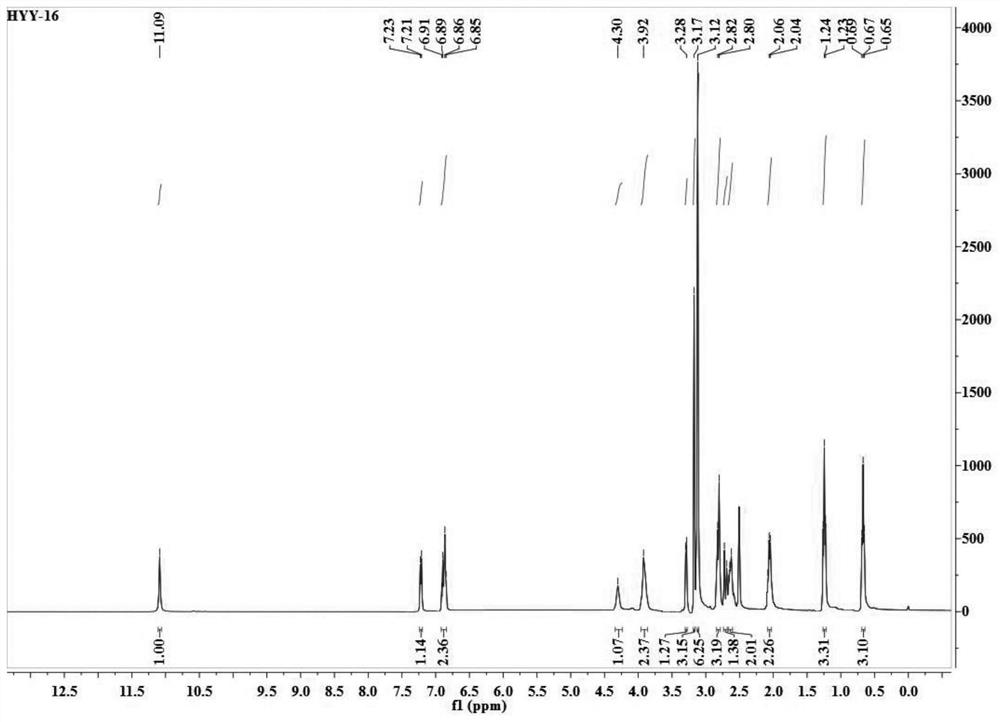
[0045] The present embodiment provides a kind of etodolac ion salt, specifically etodolac L-carnitine ion salt, which is prepared by the following steps:
[0046](1) Under an argon atmosphere, dissolve 3mmol etodolac in the reactor with 5mL of ethanol, dissolve 3mmol L-carnitine with 5mL of ethanol, and add the ethanol solution of L-carnitine dropwise to the reaction in which etodolac is dissolved. In the container, heated to 60°C, ionized modification reaction for 24 hours;
[0047] (2) After the reaction is completed, concentrate the solution to 1 / 8 of the reaction solution under vacuum conditions, freeze and crystallize, and obtain the product of etodolac L-carnitine ion salt through filtration and washing, and dry in a vacuum oven for 60 hours , to obtain pure etodolac L-carnitine ion salt.
[0048] image 3 and Figure 4 Respectively represent the proton nuclear magnetic resonance spectrum and the carbon spectrum of the etodolac L-carnitine ion salt, and the specific n...
Embodiment 3
[0050] The present embodiment provides a kind of etodolac matrine ion salt, specifically etodolac matrine ion salt, which is prepared by the following steps:
[0051] (1) Under an argon atmosphere, dissolve 3mmol etodolac in a reactor with 5mL of ethanol, dissolve 3mmol matrine with 5mL of ethanol, and add the ethanol solution of matrine dropwise to the reaction in which etodolac is dissolved. In the container, heated to 60°C, ionized modification reaction for 24 hours;
[0052] (2) After the reaction is completed, concentrate the solution to 1 / 10 of the reaction solution under vacuum conditions, freeze and crystallize, and obtain the product of etodolac matrine ionic salt through filtration and washing, and dry in a vacuum oven for 60 hours , to obtain pure etodolac matrine ion salt.
[0053] Figure 5 and Image 6 respectively represent the proton nuclear magnetic resonance spectrum and carbon spectrum of the etodolac matrine ion salt, and the specific nuclear magnetic da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com