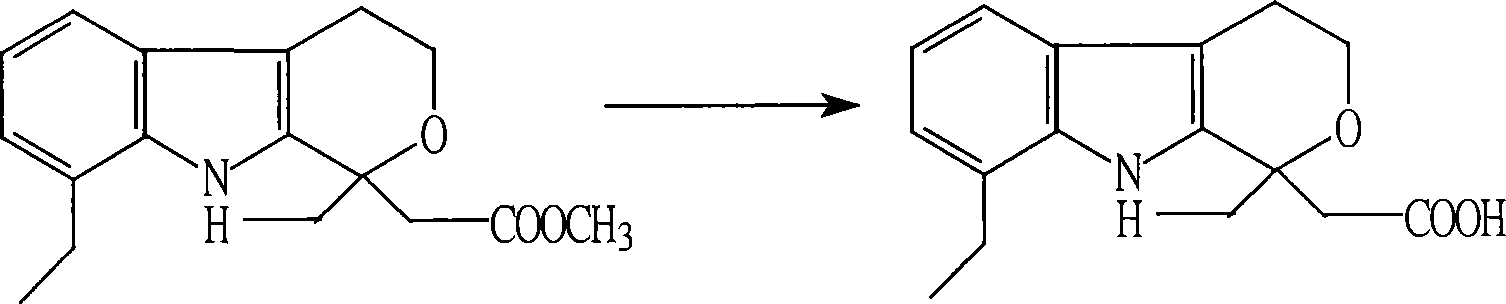Preparation method for etodolac
A technology of etodolac and methyl acetate, applied in directions such as organic chemistry, can solve the problems of unsuitability for industrial production and high cost, and achieve the effects of easy control, low production cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step A: Add 89 g of methyl etodolac, 30 ml of methanol and 150 ml of water into a 1000 ml four-necked reaction flask equipped with a stirrer and a thermometer, add 16 g of sodium hydroxide under stirring, and react at reflux with stirring for 30 minutes. After the reaction was completed, 300 ml of water was added to the reaction solution, dilute hydrochloric acid was added dropwise to pH 3-4 at room temperature, filtered and washed to obtain 84 g (yield 98.86%) of crude etodolac.
[0020] Step B: Add 84 g of crude etodolac in a 1000 ml four-necked reaction flask equipped with a stirrer and a thermometer, add aqueous sodium hydroxide solution (250 ml of water and 13 g of sodium hydroxide) while stirring, filter and wash after dissolving. The filtrate was added with 125ml ethanol, acidified with dilute hydrochloric acid to PH=3~4, filtered, washed, and dried to obtain 83g (yield 98.81%) etodolac.
Embodiment 2
[0022] Step A: Add 89 g of methyl etodolac, 30 ml of methanol and 150 ml of water into a 1000 ml four-necked reaction flask equipped with a stirrer and a thermometer, add 25 g of sodium hydroxide while stirring, and react at reflux with stirring for 30 minutes. After the reaction was completed, 300 ml of water was added to the reaction solution, dilute hydrochloric acid was added dropwise to pH 2-3 at room temperature, filtered and washed to obtain 84.5 g (yield 99.45%) of crude etodolac.
[0023] Step B: Same as Step B of Example 1.
Embodiment 3
[0025] Step A: Add 89 g of methyl etodolac, 30 ml of methanol and 210 ml of water into a 1000 ml four-necked reaction flask equipped with a stirrer and a thermometer, add 16 g of sodium hydroxide while stirring, and react at reflux with stirring for 60 minutes. After the reaction was completed, 420 ml of water was added to the reaction solution, dilute hydrochloric acid was added dropwise to pH 2-3 at room temperature, filtered and washed to obtain 84.2 g (yield 99.09%) of crude etodolac.
[0026] Step B: Same as Step B of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com