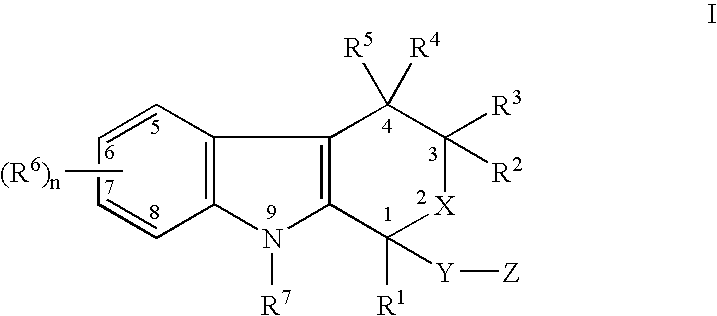
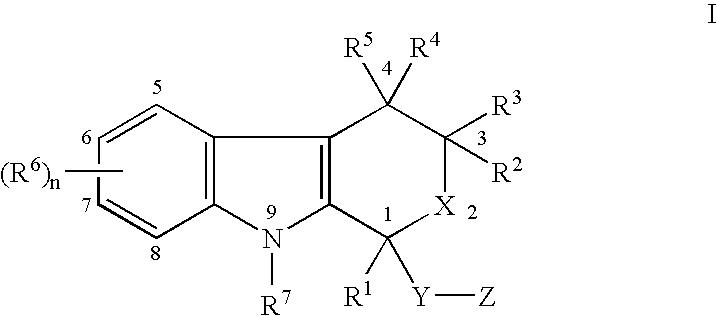
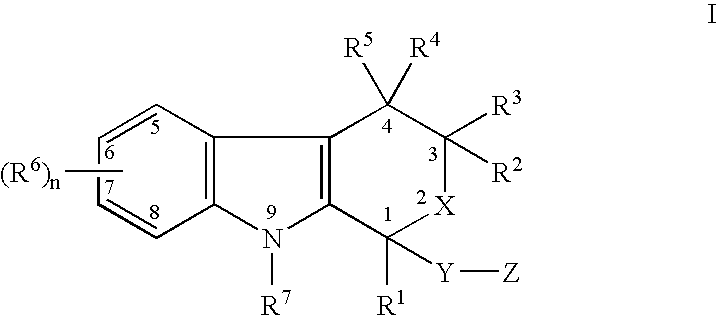
Use of etodolac to treat hyperplasia
a technology of etodolac and hyperplasia, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems that hyperplastic cells are not treated with conventional chemotherapeutic drugs, and achieve the effect of reducing the overall size of the prosta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
TRAMP Animal Model
[0068] The transgenic adenocarcinoma mouse prostate (TRAMP) mouse, was used to evaluate the effect of R-etodolac on prostate cancer progression. The etodolac was compounded into the diet at 312 ppm (low dose) and 1250 ppm (high dose) and the animals were treated for 18 weeks. At necoscopy, the urogenital system was removed and weighed. The prostate lobes, seminal vesicles, lungs, liver, and periaortic lymph nodes were preserved and sectioned for histological evaluation and graded on a 1-6 scale for degree of hyperplasia / neoplasia / carcinoma.
TABLE 1Tabel 1. Average weight of dissected prostate lobes.Weights are given as average ± standard deviationAverage weigh (g)ControlLow DoseHigh DoseAnterior Prostate0.095 ± .0520.062 ± 0.0330.076 ± 0.012Ventral prostate0.023 ± .0090.024 ± 0.0170.014 ± 0.001Lateral Prostate0.026 ± 0.110.015 ± 0.0010.016 ± 0.003Dorsal Prostate 0.169 ± 0.1670.062 ± 0.0270.053 ± 0.013Total Weigh average3.261 ± 4.9 1.804 ± 2.38 0.160 ± 0.022
[0069]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com