Etodolac double-layered osmotic pump controlled release tablets, and preparation method thereof
An osmotic pump controlled-release and etodolac technology, applied in the field of medicine, can solve the problems of easy forgetting to take medicine, low blood concentration, and inconvenience for patients, achieve lasting effective blood concentration, reduce the number of administrations, and reduce toxicity. side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Party:
[0034] Drug-containing layer (specification 100mg / tablet)
[0035] etodolac 100mg
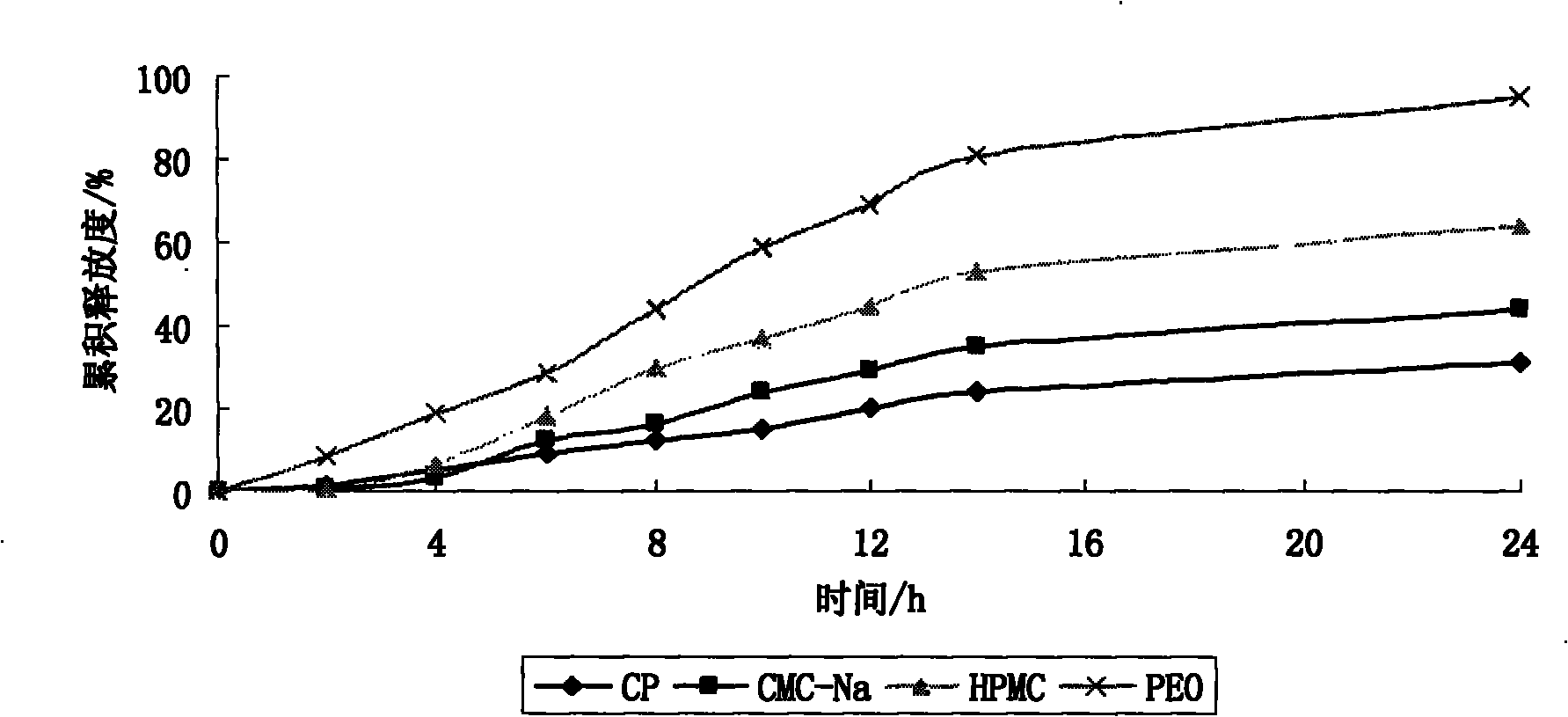
[0036] PEO (polyoxyethylene) VROH or CMC-Na (sodium carboxymethylcellulose) or HPMC K4M (Hypromellose) or CP 971NF (Carbomer)) 186mg
[0037] Booster layer (mg / tablet)
[0038] CMS-Na56
[0039] HPMC K100M 45
[0040] CP 971NF 11.6
[0041] pvp K30 twenty two
[0042] NaCl 32.5
[0043] Fe 2 o 3 1.55
[0044] Coating prescription (mg)
[0045] CA 15
[0046]PEG 3
[0047] Acetone 1000ml
[0048] In this experiment, PEO VROH (polyoxyethylene), CMC-Na (sodium carboxymethyl cellulose), HPMC K4M (Hydroxypropylmethylcellulose), CP 971NF One of the (carbomer) is used as the drug carrier of the drug-containing layer, and the influence of the polymer carrier on drug release is investigated.
[0049] Preparation method: crush etodolac and drug-containing layer auxiliary materials, pass through a 60-mesh sieve, mix well, add an app...
Embodiment 2
[0054] Party:
[0055] Drug-containing layer (specification 100mg / tablet)
[0056] etodolac 35% 100mg
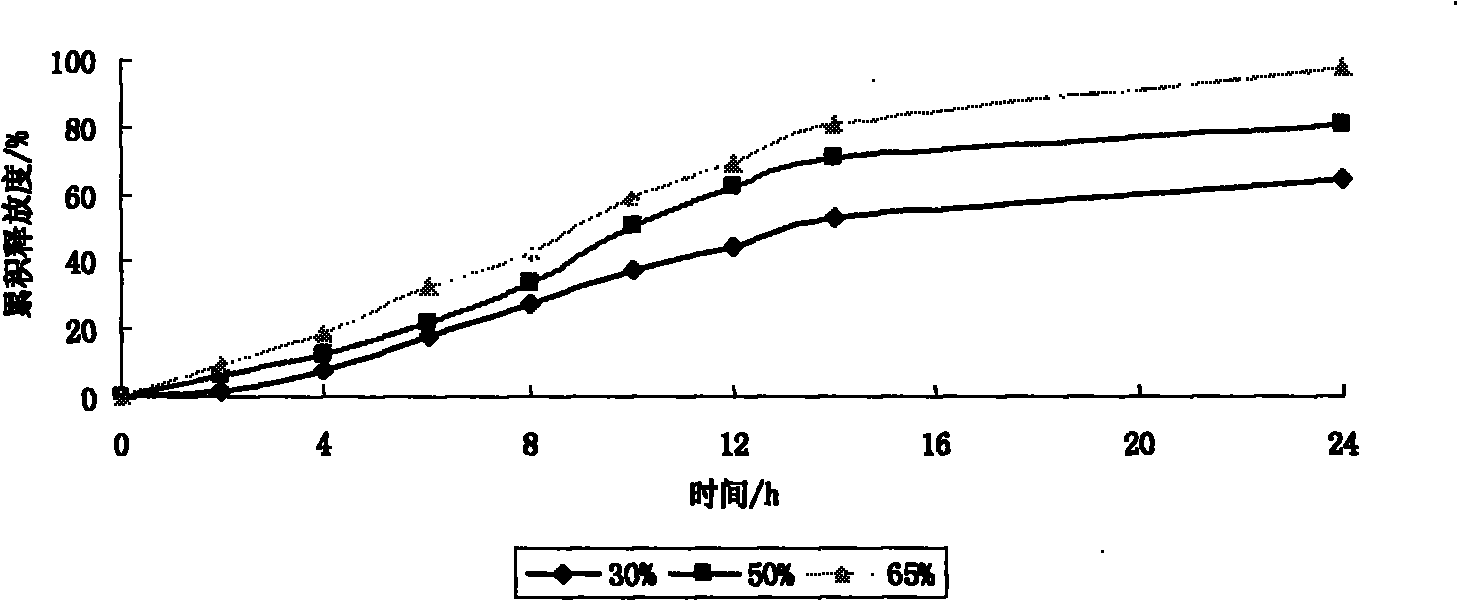
[0057] PEO VROH 30%; 50%; 65% respectively 86mg; 143mg; 186mg
[0058] Booster layer (mg / tablet)
[0059] CMS-Na56
[0060] HPMC K100M 45
[0061] CP 971NF 11.6
[0062] pvp K30 twenty two
[0063] NaCl 32.5
[0064] Fe 2 o 3 1.55
[0065] Coating prescription (mg)
[0066] CA 15
[0067] PEG 3
[0068] Acetone 1000ml
[0069] The amount of PEO used in this experiment was 30%, 50% and 65%, respectively. The release rate of the controlled-release tablets within 24 hours was measured according to the release rate method, indicating that different amounts of polyoxyethylene had an effect on the cumulative release of the drug within 24 hours.
[0070] Preparation method: crush etodolac and drug-containing layer auxiliary materials, pass through a 60-mesh sieve, mix well, use an appropriate amount of isopropanol to make a sof...
Embodiment 3
[0075] Party:
[0076] Drug-containing layer (specification 100mg / tablet)
[0077] etodolac 100mg
[0078] PEO VROH 186mg
[0079] Booster layer (mg / tablet)
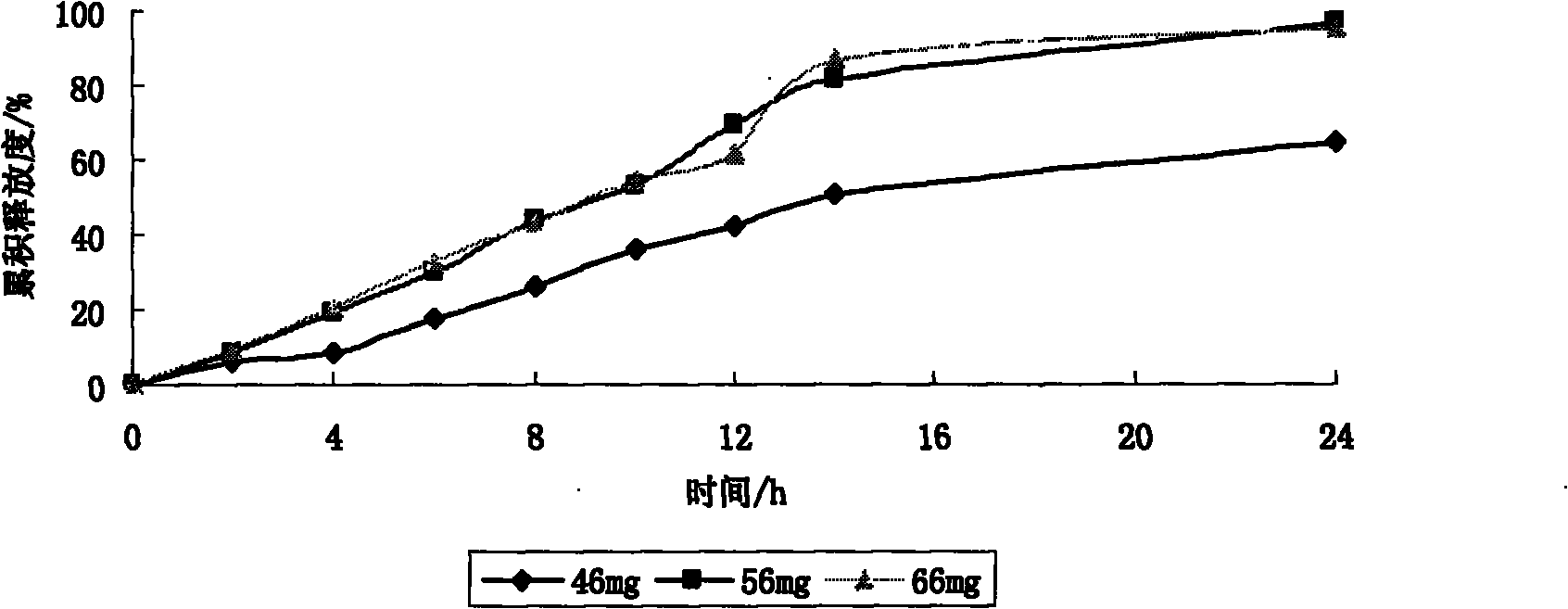
[0080] CMS-Na 46; 56; 66
[0081] HPMC K100M 45
[0082] CP 971NF 11.6
[0083] pvp K30 twenty two
[0084] NaCl 32.5
[0085] Fe 2 o 3 1.55
[0086] Coating prescription (mg)
[0087] CA 15
[0088] PEG 3
[0089] Acetone 1000ml
[0090] Keep other prescriptions of the booster layer unchanged, change the dosage of CMS-Na to 46mg, 56mg, 66mg, measure the release rate of the controlled-release tablet within 24h according to the release rate measurement method, and investigate its influence on the release rate.
[0091] Preparation method: crush etodolac and drug-containing layer auxiliary materials, pass through a 60-mesh sieve, mix well, use an appropriate amount of isopropanol to make a soft material, granulate with a 16-mesh sieve, dry at 40°C, and g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com