Novel pharmaceutical composition containing analgesic
A technology of composition and analgesic, which is applied in the field of preparation of sustained-release dosage forms, and can solve problems such as poor analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0026] Unit: mg
[0027] raw material
[0028] tramadol hydrochloride
[0029] The manufacturing method adopts general wet granulation. After sieving the drugs other than magnesium stearate and hydroxypropyl methylcellulose through a No. 35 sieve (500 μm), put them into a high-speed rotary machine and mix for 2 to 4 minutes. Then put in an appropriate amount of adhesive liquid, and after about 2-3 minutes of bonding, put it into the boiling drying machine, and dry it at a temperature of 60-70 degrees until the drying reduction is 0.5-3.0%. The dried granules are shaken and sieved with a No. 18 sieve to granulate, put into a V-shaped mixer together with magnesium stearate and mix for 3 minutes, and be compressed into tablets with a size of 4 to 8 kgf with a tablet machine.
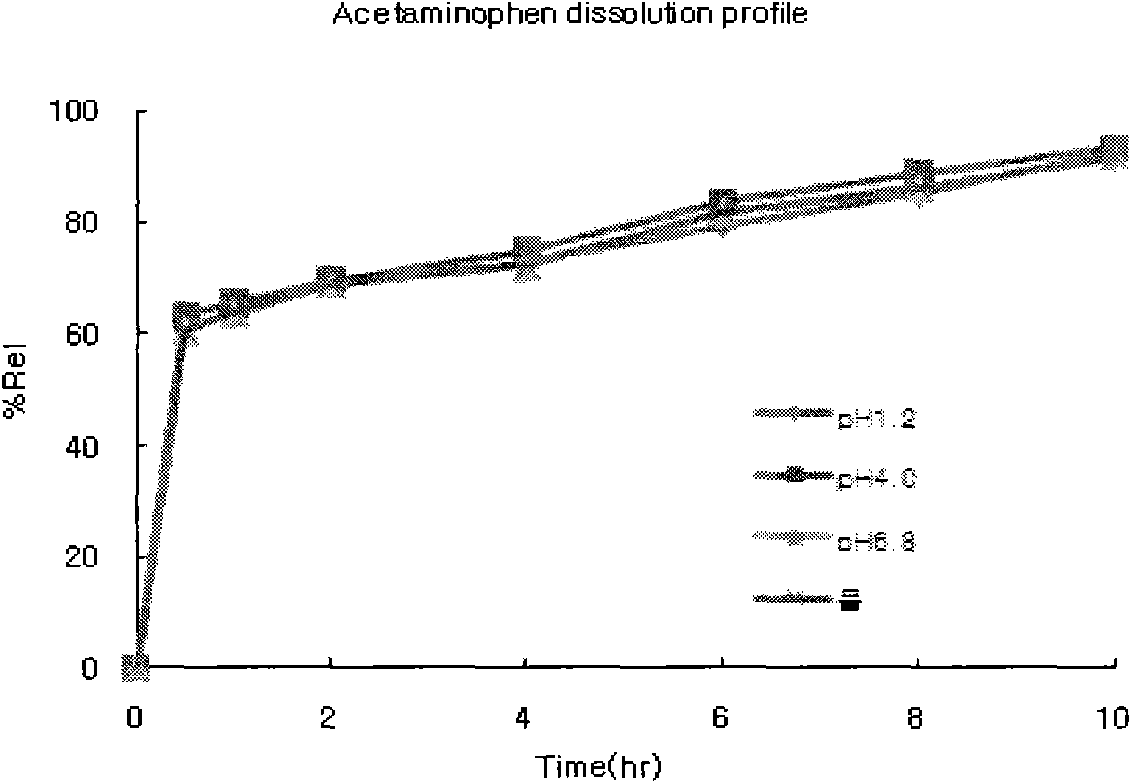
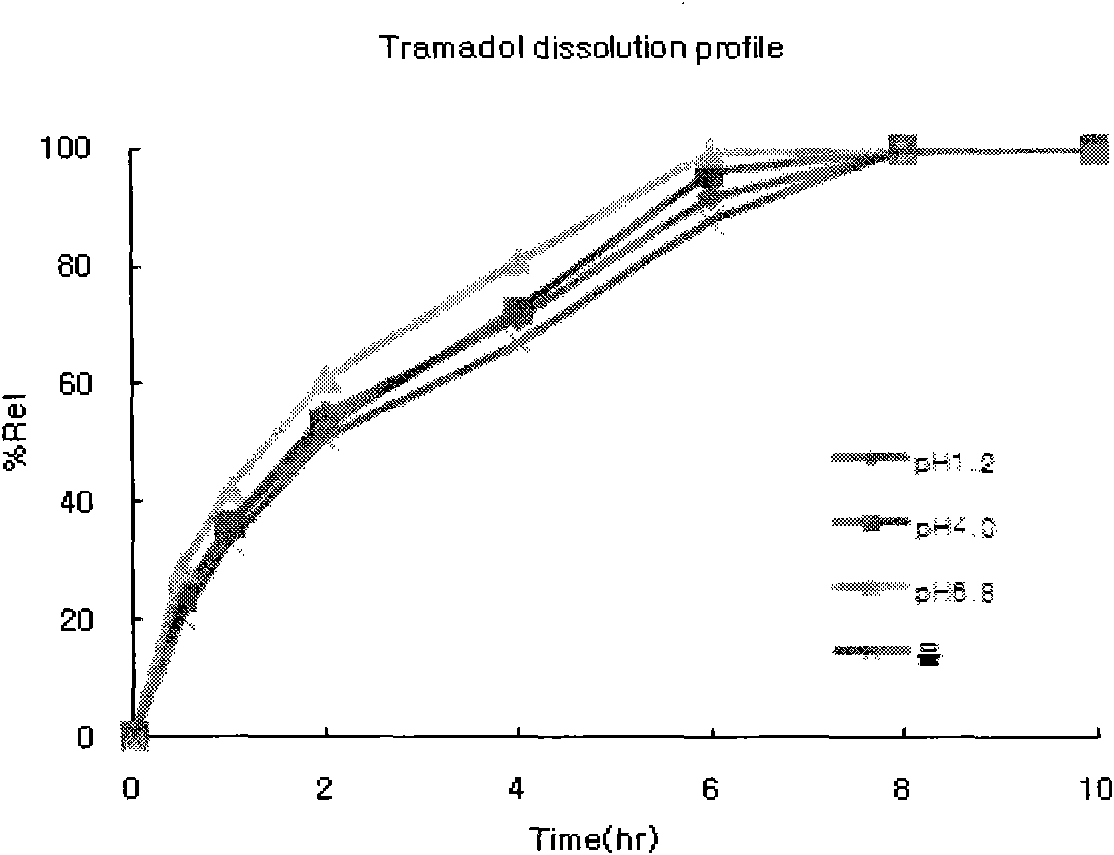
[0030] The prepared tablets were subjected to a dissolution test under the following conditions:
[0031] - Dissolution test method: Stirring method
[0032] - Eluate: .pH1.2 (900mL) ...
Embodiment 4~6
[0041] Unit: mg
[0042] raw material
Example 1
Example 2
Example 3
325.0
325.0
325.0
Silica Dispersion
3.0
3.0
3.0
15.0
15.0
15.0
Polyvinylpyrrolidone (PVP K-25)
64.5
25.0
12.0
Low-substituted hydroxypropyl cellulose L-HPC
18.1
57.6
70.6
Yellow No. 4
0.4
0.4
0.4
4.0
4.0
4.0
Total
430.0
430.0
430.0
[0043] In order to judge the manufactured tablets, the capping process was observed with the naked eye during the manufacturing process, and the dissolution test under the same conditions as the sustained-release preparation was carried out, and the release was measured when the preparation was disintegrated to more than 80%. Test results: No capping phenomenon was observed in Examples 4 and 5, but ca...
Embodiment 7~9
[0048] :mg
[0049] raw material
[0050]Test results In Examples 7 to 9, there is no decapping phenomenon, and the disintegration time of Example 9 using cross-linked polyvinylpyrrolidone is 8 minutes, which is the fastest.
[0051]
[0052] Polyvinylpyrrolidone was cross-linked using a disintegrant, and its productivity and disintegration time were evaluated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com