Oral disintegrating tablet containing tramadol hydrochloride and acetaminopher, and its preparation method
A technology of acetaminophen and tramadol hydrochloride, which is applied in the field of orally disintegrating tablets containing tramadol hydrochloride and acetaminophen and its preparation, which can solve the problems of not being able to relieve pain in time, not being able to take it in time, and having low bioavailability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 325mg / 37.5mg paracetamol tramadol / tablet, the formula is shown in Table 1.
[0033] component name
components
Amount added
325mg
tramadol hydrochloride
37.5mg
Coating Taste Masking Agent
Acrylic
15mg
Coating Taste Masking Agent
60mg
Coating Taste Masking Agent
8mg
flavoring agent
10mg
essence
lemon zest
8mg
disintegrant
Crospovidone
50mg
disintegrant
20mg
filler
70mg
filler
30mg
silica
6.0mg
0.5mg
[0034] Preparation Process:
[0035] (1) Mix 10 mg of flavoring agent aspartame and 70 mg of filler mannitol, add 0.5 mg (0.6% of the total mass of flavoring agent and ...
Embodiment 2
[0044] 325mg / 37.5mg paracetamol tramadol / tablet, the formula is shown in Table 2.
[0045] Table 2
[0046] component name
components
Amount added
325mg
[0047] active ingredient
tramadol hydrochloride
37.5mg
Coating Taste Masking Agent
Acrylic
8mg
Coating Taste Masking Agent
110mg
Coating Taste Masking Agent
15mg
flavoring agent
5mg
flavoring agent
4mg
essence
lemon zest
13mg
disintegrant
Crospovidone
46 mg
Disintegrant
10mg
filler
60mg
silica
6.1mg
0.4mg
[0048] The preparation process is as follows:
[0049] (1) Mix 5 mg of aspartame as the flavoring agent and 60 mg of the filler mannit...
Embodiment 3
[0058] 325mg / 37.5mg paracetamol tramadol / tablet. The formula is shown in Table 3.
[0059] component name
components
Amount added
active ingredient
325mg
active ingredient
tramadol hydrochloride
37.5mg
Coating Taste Masking Agent
Acrylic
12mg
Coating Taste Masking Agent
100mg
Coating Taste Masking Agent
15mg
flavoring agent
aspartame
8mg
flavoring agent
7mg
[0060] essence
11mg
disintegrant
Crospovidone
50mg
disintegrant
12mg
filler
70mg
filler
30mg
silica
6mg
0.5mg
[0061] Preparation process is with embodiment 1
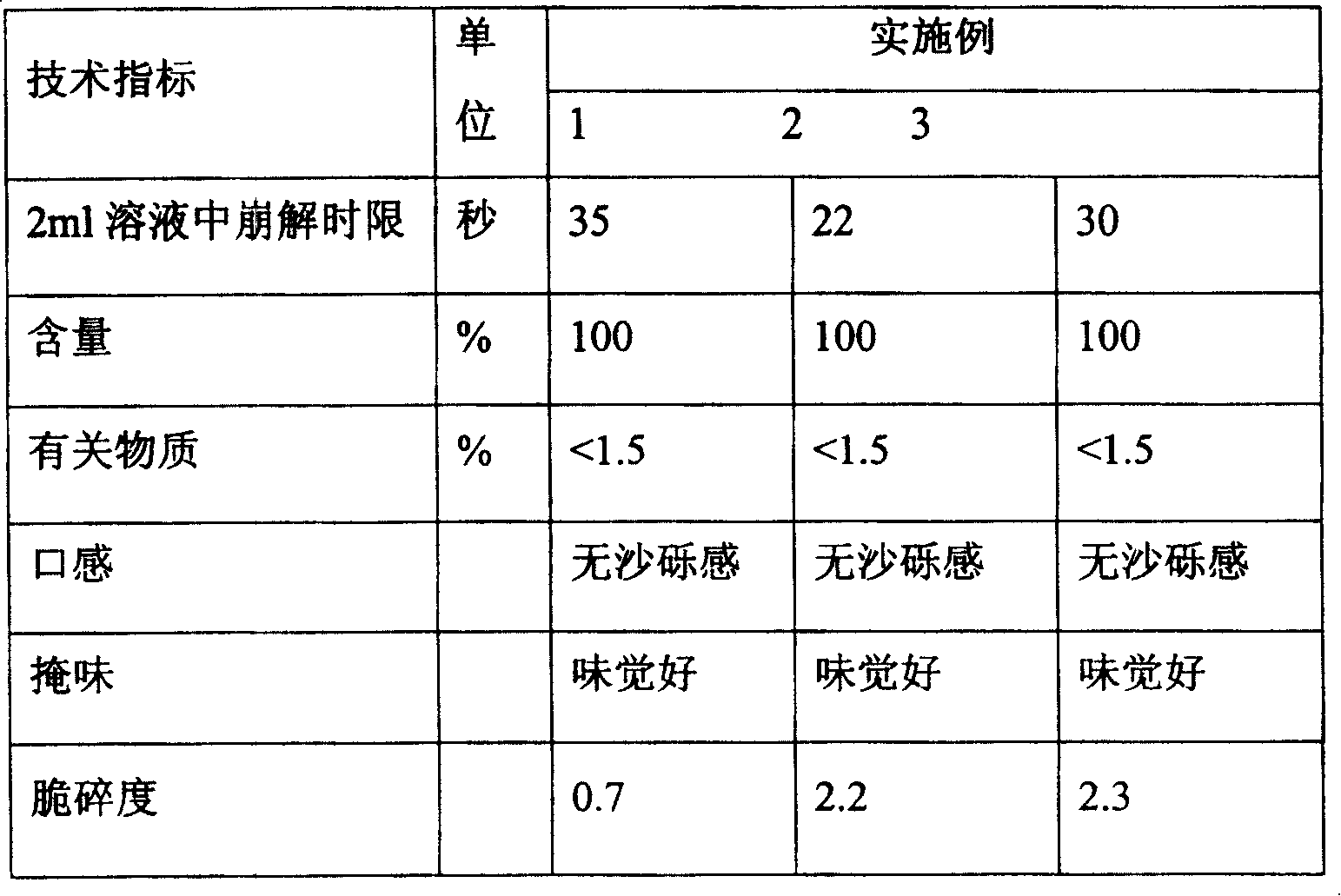
[0062] The test results are shown in Table 4.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com