Tramadol hydrochloride osmotic pump controlled release tablet
A controlled-release technology of tramadol hydrochloride and osmotic pumps, which can be used in medical preparations of non-active ingredients, organic active ingredients, nervous system diseases, etc., and can solve problems such as release performance decline and aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
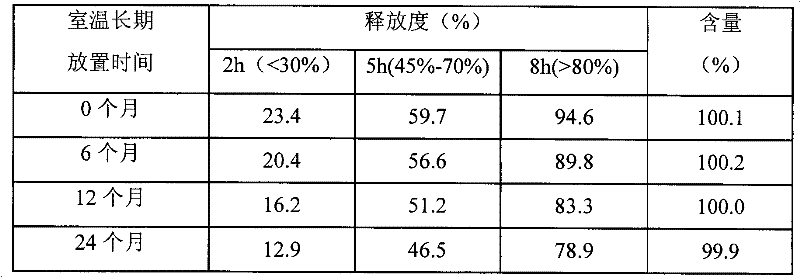
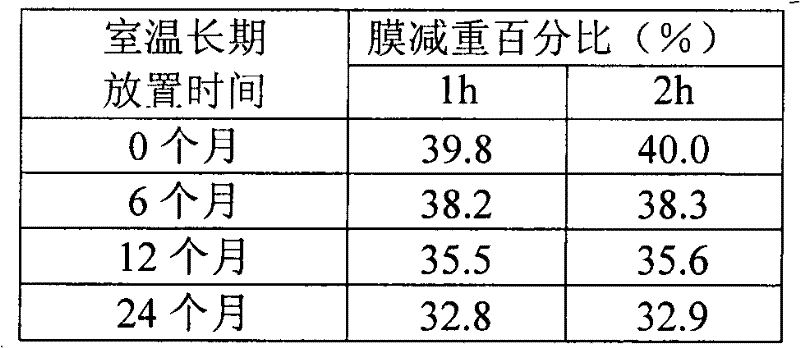
[0028] Embodiment 1: Adopt ethyl cellulose+polyethylene glycol to do the comparative embodiment of semi-permeable membrane film-forming material
[0029] 1. Prescription
[0030]1. Tablet core prescription (based on 1000 tablets)
[0031] composition
Dosage
100g
180g
microcrystalline cellulose
80g
8% povidone K30 in 70% ethanol solution
Appropriate amount
8g
[0032] 2. Semi-permeable membrane prescription
[0033] composition
Dosage
Ethylcellulose N100
30g
polyethylene glycol 4000
21g
water
100ml
900ml
[0034] 3. Film coating prescription
[0035] composition
Dosage
10g
water
100ml
[0036] 2. Preparation process
[0037] 1. Tablet core preparation
[0038] Pass tramadol hydrochloride throu...
Embodiment 2
[0066] Embodiment 2: Adopt cellulose acetate+polyethylene glycol to do the comparative example of semi-permeable membrane film-forming material
[0067] 1. Tablet core prescription: same as Example 1
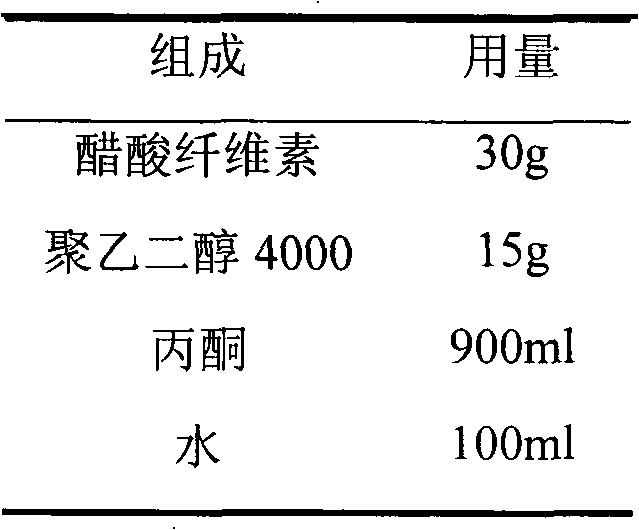
[0068] 2. Semi-permeable membrane prescription
[0069]
[0070] 3, film coating prescription: with embodiment 1
[0071] 2. Preparation process
[0072] 1. Tablet core preparation: same as Example 1
[0073] 2. Preparation process of semi-permeable membrane coating solution
[0074] Weigh the prescribed amount of polyethylene glycol 4000 and dissolve it in water, add cellulose acetate to the aqueous solution of polyethylene glycol 4000 to disperse, add the prescribed amount of acetone and stir until dissolved, to obtain.
[0075] 3. Packed with semi-permeable membrane
[0076] Put the compressed tablet into a high-efficiency coating machine and wrap it with a semi-permeable membrane, and increase the weight to 9.0%.
[0077] 4, heat treatment: with embodiment 1
[007...
Embodiment 3
[0092] Embodiment 3 The present invention adopts ethyl cellulose+povidone to do the embodiment of semi-permeable membrane film-forming material
[0093] 1. Prescription
[0094] 1. Tablet core prescription: same as Example 1
[0095] 2. Semi-permeable membrane prescription
[0096] prescription
Dosage
Ethylcellulose N100
30g
Povidone K30
18g
1000ml
[0097] 3, film coating prescription: with embodiment 1
[0098] 2. Preparation process
[0099] 1. Tablet core preparation: same as Example 1
[0100] 2. Preparation process of semi-permeable membrane coating solution
[0101] Weigh the prescribed amount of ethyl cellulose and add it to ethanol, stir until dissolved, then add the prescribed amount of povidone and stir until completely dissolved, to obtain.
[0102] 3. Packed with semi-permeable membrane
[0103] Put the compressed tablet into a high-efficiency coating machine and wrap it with a semi-permeabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com