Composite tramadol hydrochloride formulation and preparation process thereof
A technology of tramadol hydrochloride and preparations, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as affecting the quality of life of patients, difficult to achieve curative effects, addiction, and toxic and side effects. , to achieve the effect of being easy to take and transport, reducing addiction, and taking effect quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
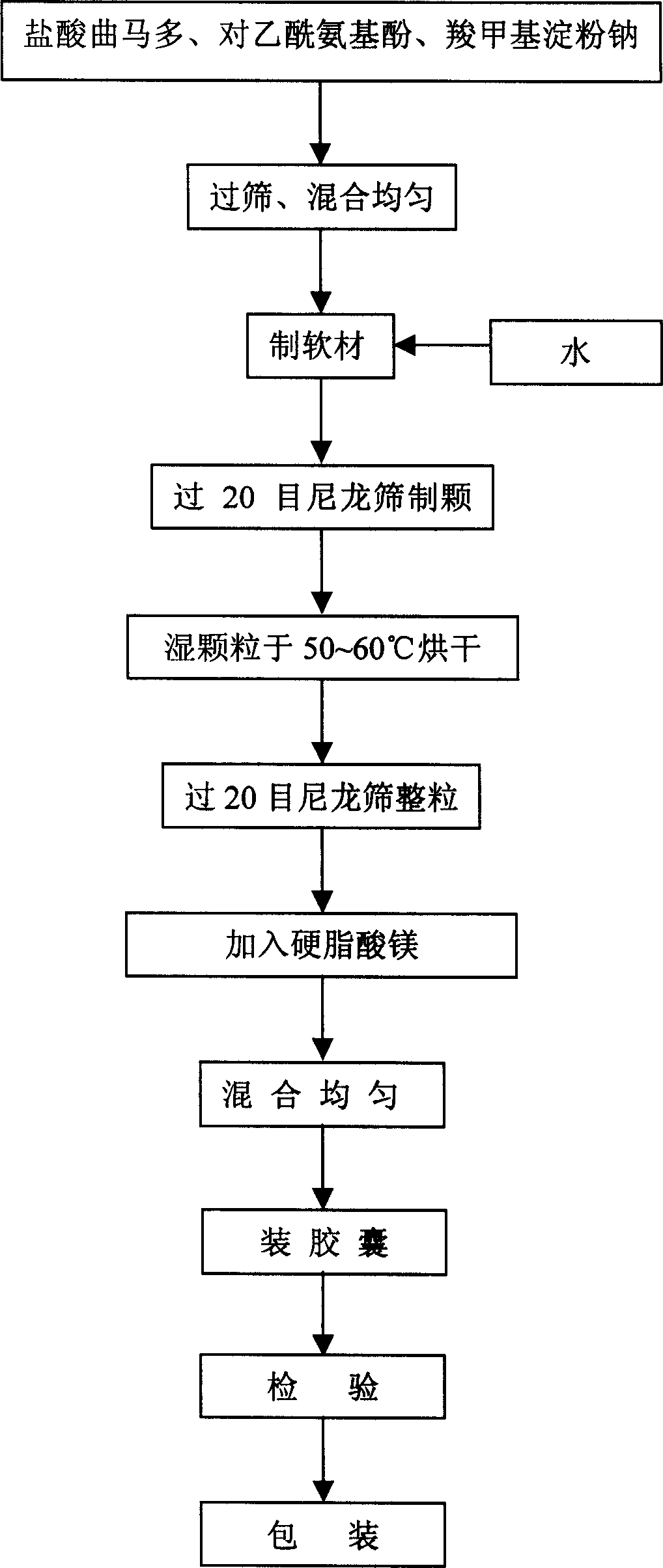
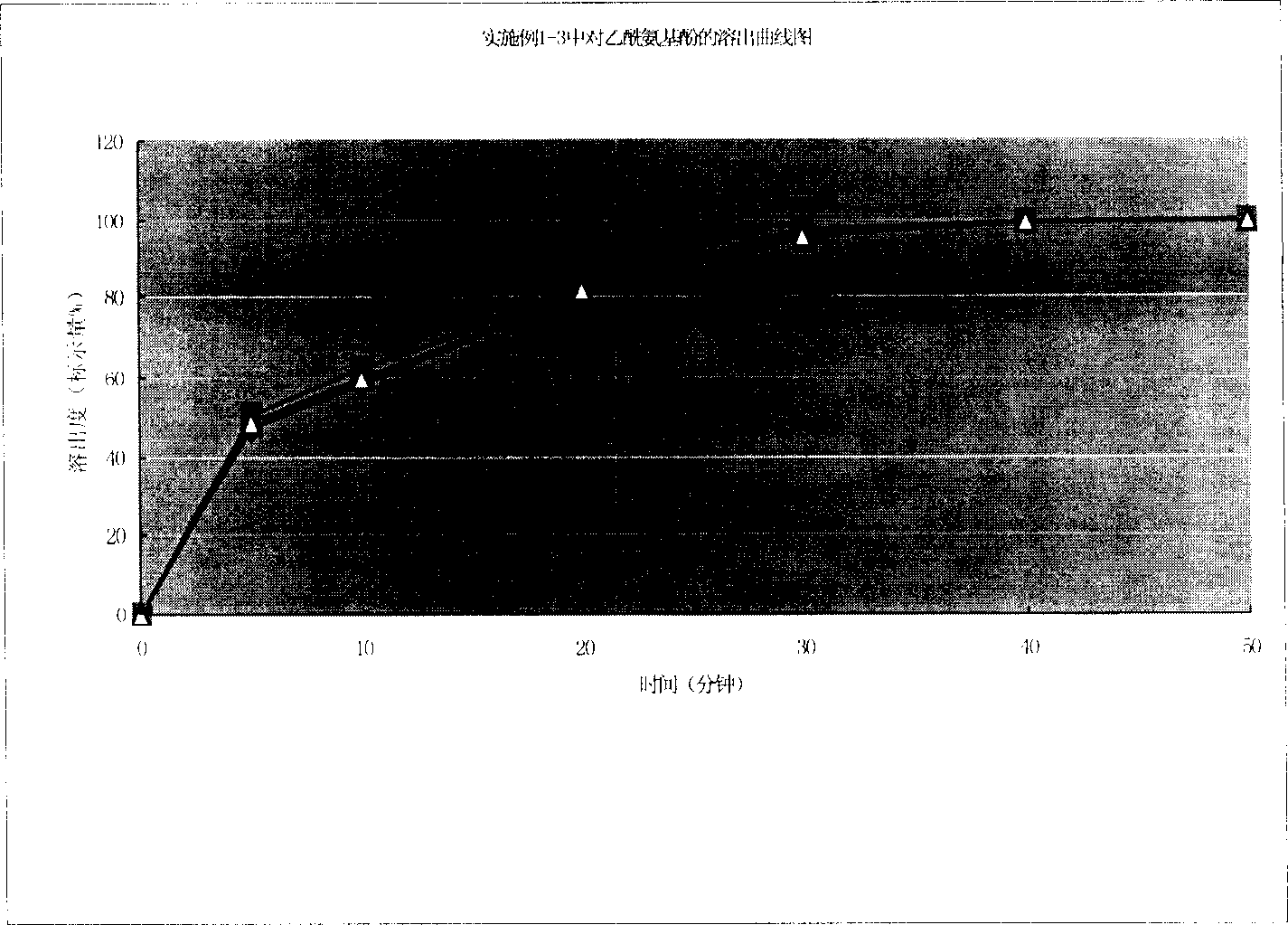
[0074] Pass acetaminophen (Huzhou Kangquan Pharmaceutical Co., Ltd.), tramadol hydrochloride (Shandong Xinhua Pharmaceutical Co., Ltd.) and sodium carboxymethyl starch (Huzhou Food and Chemical United Company) respectively through a 100-mesh sieve to get tramadol hydrochloride Add 42g of paracetamol, 325g of acetaminophen, and 7.5g of sodium carboxymethyl starch, mix well, add appropriate amount of water, make soft material, pass through a 20-mesh sieve to make wet granules, blow dry at 50°C, and use a 20-mesh sieve Whole grain, add magnesium stearate (Huzhou Food Chemical United Company) 2.5g, measure content, calculate filling amount, fill No. 0 capsule, pack, put in storage, check, get 1000 capsules of finished product. Each capsule contains tramadol hydrochloride 42mg and acetaminophen 325mg. Capsules can better cover the bitter taste of the main drug, release faster, take effect quickly, provide patients with more choices in use, and are easy to take and transport.
Embodiment 2
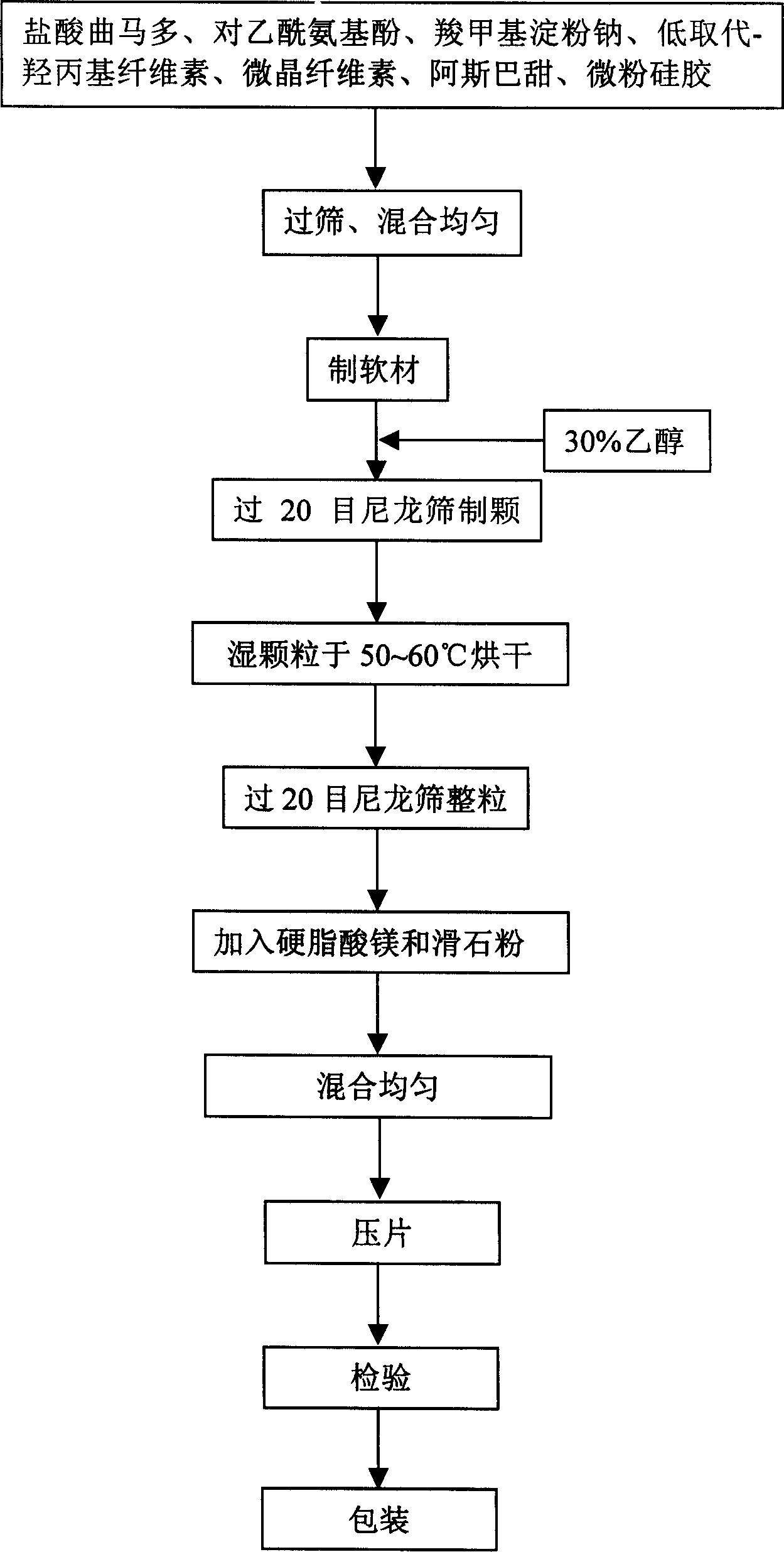
[0076] Pass paracetamol, tramadol hydrochloride and carboxymethyl starch sodium through 100 mesh sieves respectively, take tramadol hydrochloride 40g, paracetamol 350g, carboxymethyl starch sodium 7.5g, fully mix, add water appropriate amount, prepare Make soft materials, pass through a 20-mesh sieve to make wet granules, blow dry at 55°C, granulate with a 20-mesh sieve, add 2.5g of magnesium stearate, measure the content, calculate the filling amount, fill the No. 0 capsule, Packing, warehousing, inspection, and 1000 finished capsules were obtained.
Embodiment 3
[0078] Pass paracetamol, tramadol hydrochloride and carboxymethyl starch sodium through 100 mesh sieves respectively, take tramadol hydrochloride 45g, paracetamol 300g, carboxymethyl starch sodium 7.5g, fully mix, add water appropriate amount, prepare Make soft materials, pass through a 20-mesh sieve to make wet granules, blow dry at 60°C, granulate with a 20-mesh sieve, add 2.5g of magnesium stearate, measure the content, calculate the filling amount, fill the No. 0 capsule, Packing, warehousing, inspection, and 1000 finished capsules were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com