Trimetazidine hydrochloride double-layer osmotic pump controlled-release tablet and preparation method
A technology for controlled release of trimetazidine hydrochloride and osmotic pump, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve uneven drug release, poor release rate control, etc. problems, to prolong the action time in the body, maintain stability, and reduce the number of times of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
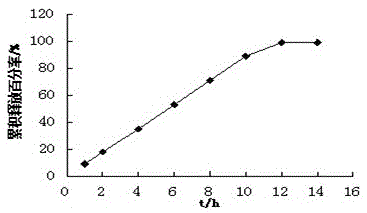
example 1
[0023] Tablet prescription:
[0024] Drug-containing layer
[0025] Trimetazidine Hydrochloride 35mg(29.9%)
[0026] Polyoxyethylene N80 80mg (68.4%)
[0027] Magnesium Stearate 2mg (1.7%)
[0028] booster layer
[0029] Polyoxyethylene WSR303 60mg (73.2%)
[0030] Sodium chloride 20mg (24.4%)
[0031] Magnesium Stearate 2mg (2.4%)
[0032] Coating film prescription:
[0033] Cellulose acetate 30g(88.2%)
[0034] Polyethylene glycol 4g (11.8%)
[0035] Solvent Prescription for Dissolving Coating Material
[0036] Acetone 1000mL
[0037] Distilled water 15mL
[0038] Preparation Process:
[0039] Pass the drug, polyoxyethylene and magnesium stearate in the prescribed amount of the drug-containing layer through an 80-mesh sieve, and mix evenly; pass the prescribed amount of polyoxyethylene, sodium chloride and magnesium stearate in the booster layer through an 80-mesh sieve, and mix Evenly, add the drug-containing layer granules and the booster layer granules sequen...
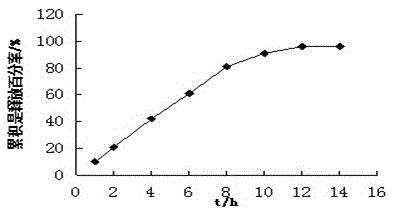
example 2
[0041] Tablet prescription:
[0042] Drug-containing layer
[0043] Trimetazidine Hydrochloride 35mg(40.2%)
[0044] Polyoxyethylene N80 50mg (57.5%)
[0045] Talc 2mg (2.3%)
[0046] booster layer
[0047] Polyoxyethylene WSR301 60mg (37.0%)
[0048] Sodium Chloride 100mg (61.7%)
[0049] Talc 2mg (1.3%)
[0050] Coating film prescription:
[0051] Ethylcellulose 30g (85.7%)
[0052] Polyethylene glycol 5g (14.3%)
[0053] Solvent Prescription for Dissolving Coating Material
[0054] Acetone 1000mL
[0055] Distilled water 15mL
[0056] Preparation Process:
[0057] Pass the drug, gum arabic and talcum powder in the prescription amount of the drug layer through an 80 mesh sieve, and mix evenly; pass the polyoxyethylene, sodium chloride and talcum powder in the prescription amount of the booster layer through an 80 mesh sieve, and mix evenly, and the drug-containing layer The granules and the booster layer granules are sequentially added into the 7 mm shallow concav...
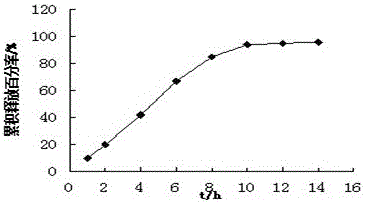
example 3
[0059] Tablet prescription:
[0060] Drug-containing layer
[0061] Trimetazidine Hydrochloride 35mg (27.6%)
[0062] Sodium Alginate 90mg (70.9%)
[0063] Micronized silica gel 2mg (1.5%)
[0064] booster layer
[0065] Polyoxyethylene WSR Coagulant 60mg (83.3%)
[0066] Sodium Chloride 10mg (13.9%)
[0067] Micronized silica gel 2mg (2.8%)
[0068] Coating film prescription:
[0069] Cellulose acetate 30g (83.3%)
[0070] Hypromellose 6g (16.7%)
[0071] Solvent Prescription for Dissolving Coating Material
[0072] Acetone 1000mL
[0073] Distilled water 15mL
[0074] Preparation Process:
[0075] Pass the drug, sodium alginate, and micropowder silica gel in the prescription amount of the medicine layer through an 80-mesh sieve, and mix evenly; Layer granules and booster layer granules are sequentially added to a 7 mm shallow concave punch, and pressed to form a double-layer osmotic pump core. Dissolve cellulose acetate and hydroxypropyl methylcellulose in acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com