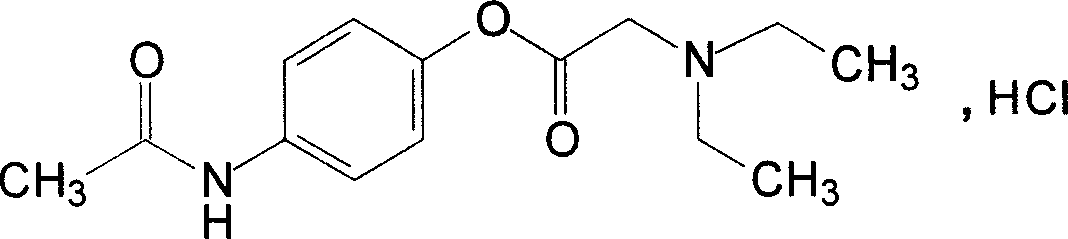
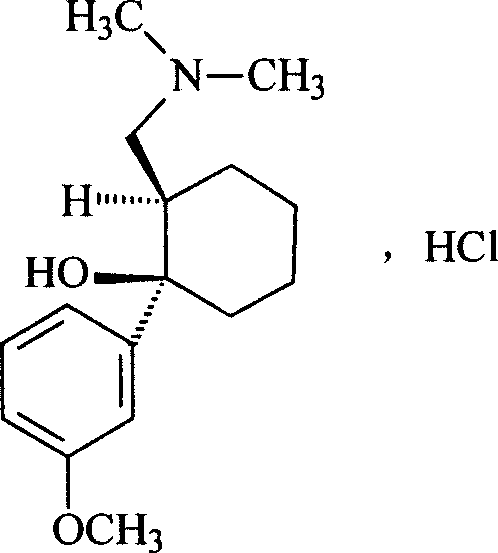
Pharmaceutical composition for injection and its medicine box
A composition and injection technology, which can be used in drug combinations, antipyretics, nervous system diseases, etc., and can solve problems such as strong vascular stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Preparation of samples
[0026] 1) Preparation of tramadol hydrochloride and propadamol hydrochloride mixture
[0027] Commercially available tramadol hydrochloride and propadamol hydrochloride were used, and according to general injection preparation requirements, the ratio of tramadol hydrochloride 37.5 mg and propadamol hydrochloride 642.5 mg was prepared into sterile powder for injection for later use.
[0028] 2) Sodium citrate solution
[0029] According to the general injection solution preparation method, prepare 2.5% sodium citrate injection solution for later use.
[0030] 3) Mix the sample prepared in step 1 with 5 ml of 2.5% sodium citrate injection solution for later use.
Embodiment 2
[0031] Example 2. Preparation of the kit
[0032] 1) According to the general injection preparation method, each bottle contains 37.5 mg of tramadol hydrochloride and 642.5 mg of propatamol hydrochloride to prepare a sterile powder for injection as one of the bottles.
[0033] 2) According to the general injection preparation method, configure 0.125g / 5ml of sodium citrate injection as another bottle.
[0034] 3) Pack both bottles in the same box. In use, the sodium citrate solution is injected into the bottles of tramadol hydrochloride and propatamol hydrochloride, and after being dissolved, it is diluted with a solvent for injection.
Embodiment 3
[0035] Example 3. Vascular irritation test
[0036] 1. Test material
[0037] 1.1 Test substance: Take the kit of Example 2, dissolve tramadol hydrochloride and propatamol hydrochloride in the sodium citrate solution therein, and then mix with 100 0.9% normal saline to make a solution.
[0038] Control: in the kit of Example 2, only tramadol hydrochloride and propatamol hydrochloride were used, and were mixed with 100 0.9% normal saline to prepare a solution.
[0039] 1.2 Dosage setting: the administration volume of test substance and control is 10ml / kg.
[0040] 1.3 Animals: rabbits, Japanese large-eared whites, weighing 2.4-3.0 kg, provided by Shenyang Shuangyi Laboratory Animal Research Institute.
[0041] Laboratory animal production license number: SCXK (Liao) 2003-0012. Laboratory animal use license number:
[0042] SYXK (Liao) 2003-0024.
[0043] 1.4 Test conditions: temperature: 22~24℃, relative humidity: 34~50%
[0044] 2. Test methods and results
[0045] Five...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com