Method for determination of related substances of paracetamol and tramadol hydrochloride capsules
A technology of related substances and determination methods, which is applied in the field of determination of related substances of paracetamol tramadol capsules, to achieve the effects of improving detection ability, quality control, and scientific and reasonable methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1 Experimental instruments, materials and reagents
[0029] Sample: Amphenol tramadol capsules (batch numbers: 140601, 140602, 140603), provided by Shanxi Good Doctor Pharmaceutical Co., Ltd.;
[0030] Reference substances: paracetamol (batch number: 100018-200408), tramadol hydrochloride (batch number: 171242-201005), cis-trama hydrochloride (batch number: 171255-201103), p-aminophen (batch number: 100802-201002) , from China National Institute for the Control of Pharmaceutical and Biological Products, China National Institute for Food and Drug Control;
[0031] Reagents: tetrahydrofuran is chromatographically pure, triethylamine, phosphoric acid and potassium dihydrogen phosphate are analytically pure, and water is self-made ultrapure water;
[0032] Instruments: Agilent 1200 high performance liquid chromatography (Agilent), LC-2010CHT high performance liquid chromatography (Shimadzu, Japan), electronic balance Mettler XS-205.
[0033] 2 Experimental part
[0034] ...
experiment example 1
[0048] Experimental Example 1 Determination of Related Substances in Tramadol Capsules and Screening of Chromatographic Conditions
[0049] Basic chromatographic conditions: octylsilane bonded silica gel as filler, 0.005M potassium dihydrogen phosphate (containing 0.1% phosphoric acid and 0.1% triethylamine)-tetrahydrofuran (95:5) as mobile phase, flow rate 1.0ml / min, The column temperature is 35°C.
[0050] (1) Detection wavelength selection
[0051] With reference to the detection wavelength 216nm of related substances in the paracetamol tramadol tablets in the USP United States Pharmacopoeia (36), 215nm was selected as the detection wavelength. Although the solvent peak detected at 215nm wavelength is more obvious, it does not interfere with the detection of related substances, and it is also conducive to the detection of impurities with only terminal ultraviolet absorption. Therefore, the detection wavelength of 215nm is determined to be selected.
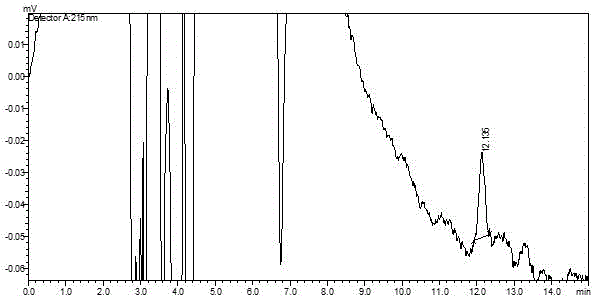
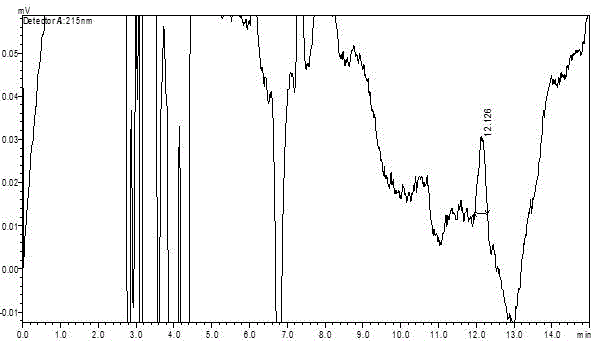
[0052] (2) Specificity ...
experiment example 2
[0063] Experimental Example 2 Methodological Investigation of Determination of Related Substances in Tramadol Capsules
[0064] 2.1 Investigation on detection method of cis-tramadol hydrochloride
[0065] ① Linear inspection
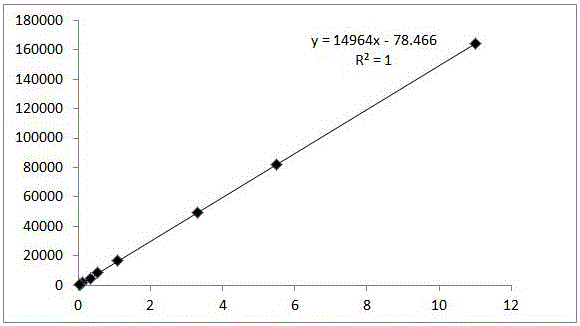
[0066] Take an appropriate amount of cis-tramadol hydrochloride reference substance, accurately weigh it, add methanol-water (1:9) to dissolve and make the concentration 11 μg / ml, 5.5 μg / ml, 3.3 μg / ml, 1.1 μg / ml, 0.55 μg / ml, 0.33μg / ml, 0.11μg / ml, 0.055μg / ml and 0.0275μg / ml serial standard curve solution. Precisely measure 10 μl of the linear series of solutions and inject them into the liquid chromatograph respectively, record the chromatogram, take the peak area as the ordinate, and the concentration as the abscissa, and the linear equation obtained by regression is y=14953x-61.488, R 2 =1; the linear range is 0.0275-11.007 μg / ml. The results are shown in Table 1 and Figure 8 .
[0067] Table 1 Linear Data Table
[0068]
[0069]
[0070] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com