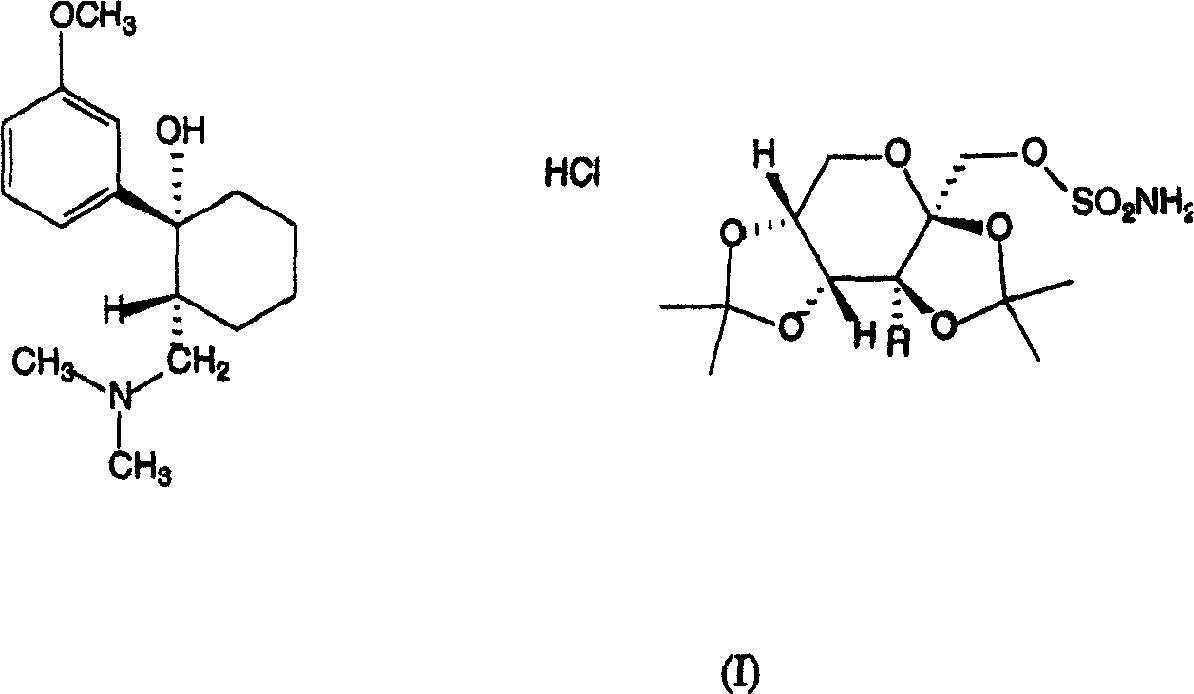
Adduct of topiramate and tramadol hydrochloride and uses thereof
A technology for tramadol hydrochloride and adducts, which is applied to the preparation of sugar compounds with non-glycosyl groups, organic compounds, active ingredients of heterocyclic compounds, etc., and can solve the problems of long half-life and short half-life of topiramate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the preparation of formula (I) adduct
[0051] Mix 2 moles of tramadol hydrochloride and 1 mole of topiramate in ethanol, heat to 40°C, and keep the temperature for a sufficient time until the solid drug is completely dissolved. The mixture is then cooled to a temperature of about 2-8°C, whereupon crystallization begins. The whole mixture was left at this temperature for several hours to form the remaining crystals. The entire material was filtered cold and the crystals dried. The resulting 1:1 adduct had a melting point of about 161°C (onset temperature).
Embodiment 2
[0052] Example 2: Composition Example
[0053] Typical pharmaceutical compositions of the invention in dosage unit form suitable for systemic or topical administration to warm-blooded animals are illustrated below.
[0054] capsule
[0055] 50 g of the adduct of formula (I), 12 g of sodium lauryl sulfate, 112 g of starch, 112 g of lactose, 1.6 g of colloidal silicon dioxide, and 2.4 g of magnesium stearate were stirred vigorously. The resulting mixture is then filled into 1000 suitable hard gelatine capsules, each containing 50 mg of the adduct of formula (I).
[0056] Film Coated Tablets
[0057] Preparation of tablet cores
[0058] 500g formula (I) adduct, the mixture of 2850g lactose and 1000g starch is mixed completely, and mixture is mixed with 25g sodium lauryl sulfate and 50g polyvinylpyrrolidone (Kollidon-K 90 TM ) in about 1000ml of water to wet the solution. The wet powder mixture is sieved, dried and re-sifted. Then add 500g microcrystalline cellulose (Avicel)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com