Co-crystals of tramadol and nsaids
A technology of tramadol and heterocycle, applied in the field of co-crystal of tramadol and NSAID, can solve the problems such as little known
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0300] Example 1a: Method for obtaining (-)-tramadol-(S)-naproxen (1:2) co-crystal
[0301]A solution of (S)-naproxen (2.14 g, 9.3 mmol) in 20 mL of methanol was added to (-)-tramadol (2.45 g, 9.3 mmol) in 10 mL of methanol over 10 minutes with stirring in solution. The resulting solution was stirred at room temperature for 30 minutes, and the solvent was evaporated in vacuo to a pale yellow oil. The oil was cooled to -197°C and then warmed to room temperature to afford amorphous (-)-tramadol-(S)-naproxen salt as a white solid (4.59 g, 100%).
[0302] Procedure: Suspend the amorphous (-)-tramadol-(S)-naproxen salt (1:1) (2.2 g, 4.46 mmol) obtained above in 10 mL of diisopropyl ether and heat at room temperature Under stirring for 7 days. The resulting suspension was filtered off. The filtrate was washed with about 2 mL of diisopropyl ether and dried under vacuum (10 mm Hg) at 40 °C for 24 hours to obtain a 1:2 ratio of (-)-tramadol-(S)-naproxen co- crystalline as a crys...
Embodiment 1b
[0303] Example 1b: Method for obtaining (-)-tramadol-(S)-naproxen co-crystal (1:2) co-crystal:
[0304] A solution of (-)-tramadol (0.58 g, 2.20 mmol) in 2 mL of isopropanol was added to (S)-naproxen (1.02 g, 4.43 mmol, 2 eq) in 2 mL of Stir the suspension in isopropanol. The resulting solution was cooled to room temperature and one-third of the solvent was evaporated. 5-10 mg of crystalline (-)-tramadol-(S)-naproxen co-crystal (1:2) was added as a seed crystal to the solution, and it was mixed at room temperature without stirring. Let stand for 48 hours. The resulting suspension was filtered off and the filtrate was washed with about 1 mL of isopropanol and dried under vacuum (10 mm Hg) at 60°C for 24 hours to obtain a co-crystal (-)-tramadol-(S) in a 1:2 ratio - Naproxen as a white solid (1.31 g, 81%).
[0305] Characterization of the cocrystal:
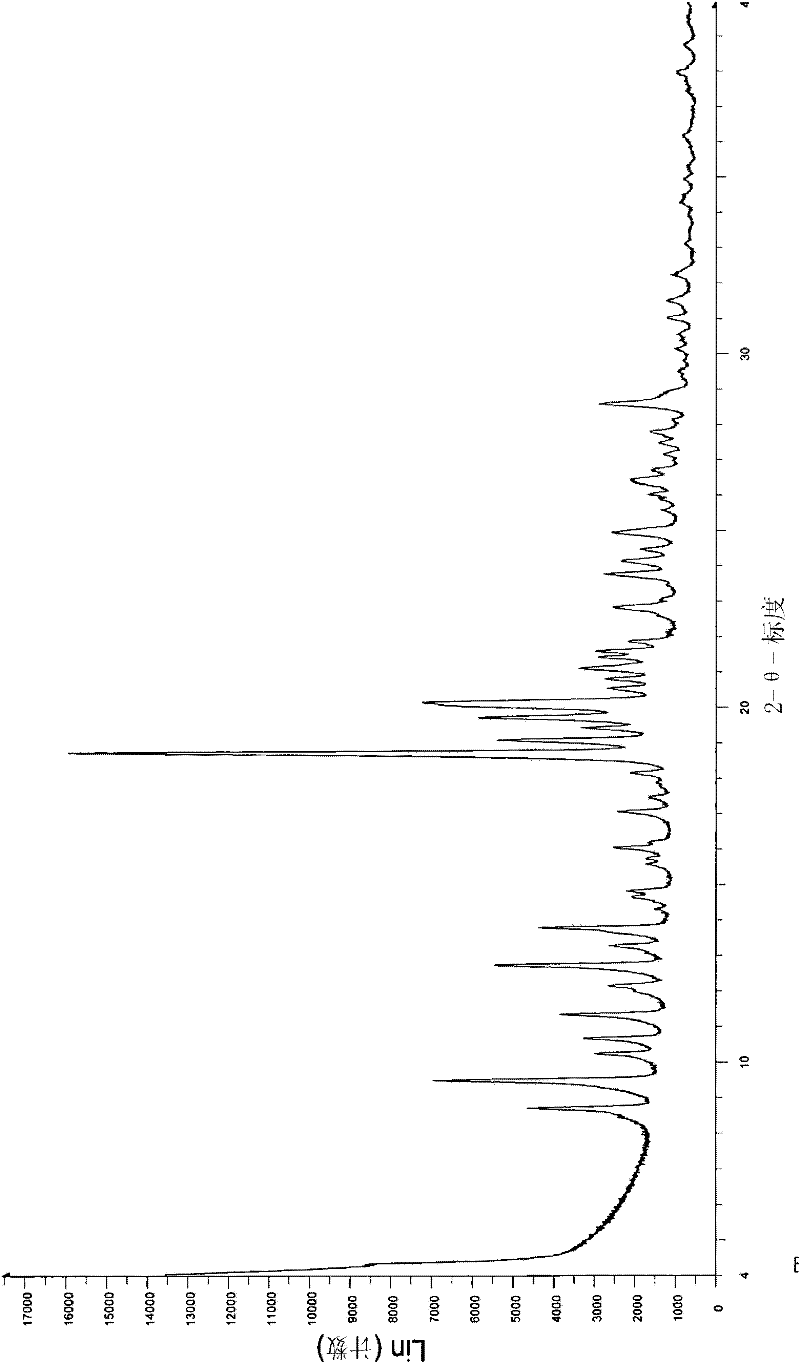
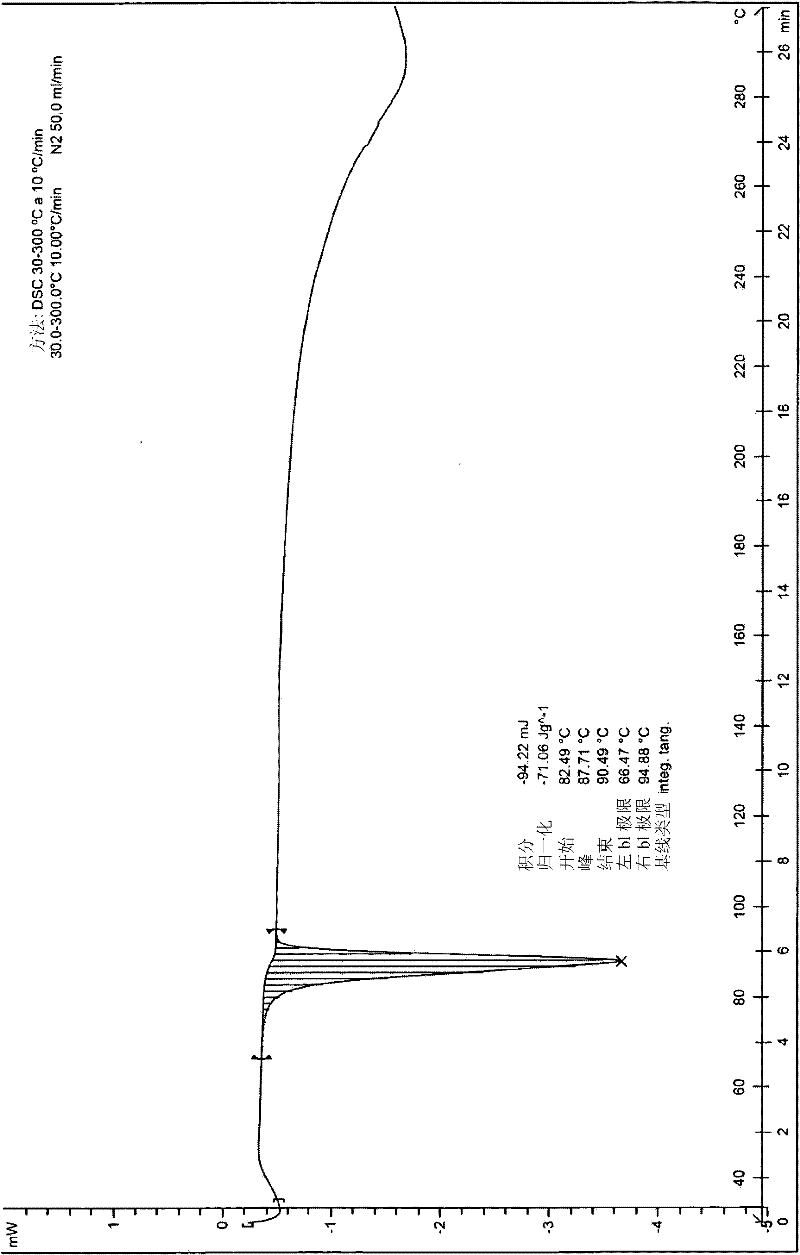
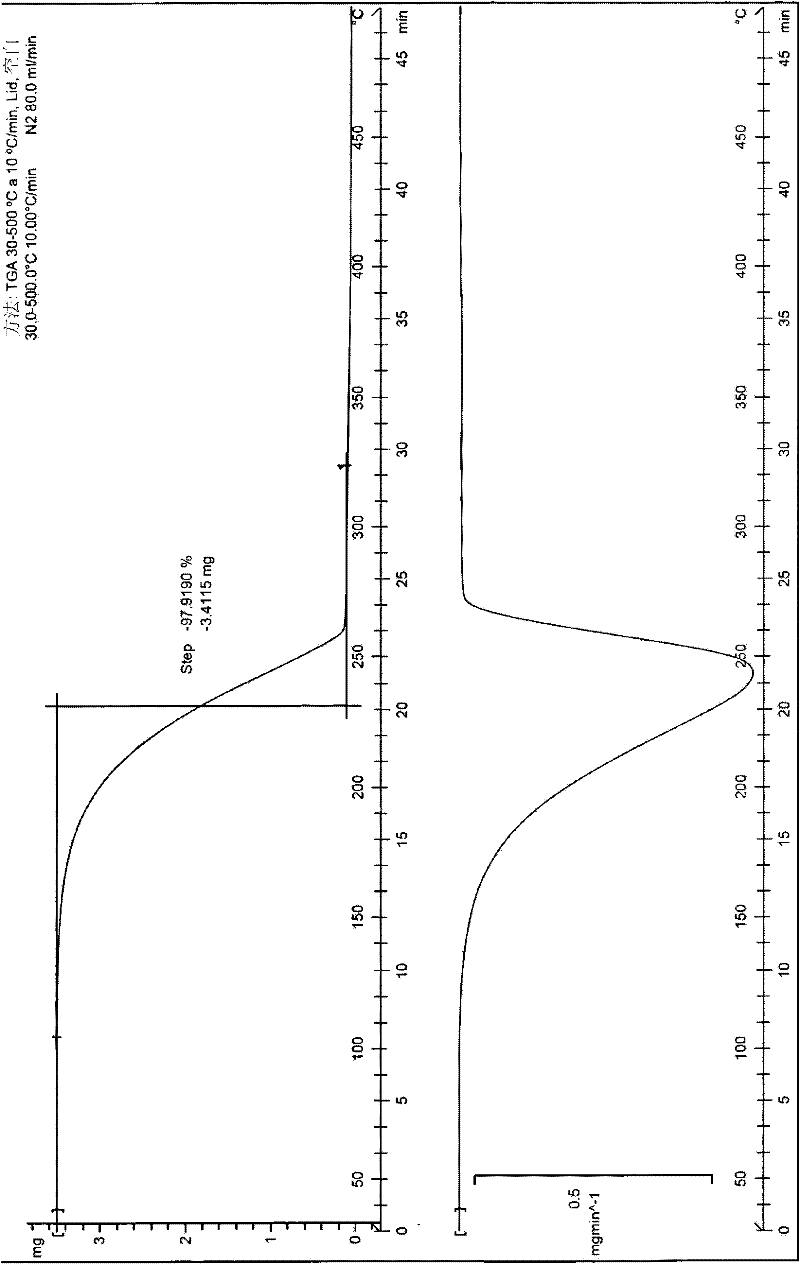
[0306] The (-)-tramadol-(S)-naproxen (1:2) co-crystal obtained according to Example 1 was tested by 1H-NMR, FTIR, powder...
Embodiment 2
[0328] Example 2: (+)-Tramadol-(R)-Naproxen (1:2) Cocrystal
[0329] Method for obtaining (+)-tramadol-(R)-naproxen (1:2) co-crystal
[0330] A solution of (R)-naproxen (751 mg, 3.26 mmol) in 4 mL of methanol was added to a solution of (+)-tramadol (430 mg, 1.63 mmol) in 1 mL of methanol. The mixture was stirred for 30 minutes and the solvent was evaporated in vacuo to an oil which solidified by cooling to -197°C. The resulting solid was suspended in 10 mL of diisopropyl ether and stirred at room temperature for 7 days. The resulting suspension was filtered off. The filtrate was washed with 5 mL of diisopropyl ether and dried under vacuum (10 mm Hg) at 40° C. for 16 hours to obtain a cocrystal of (+)-tramadol-(R)-naproxen in a 1:2 ratio, It was a crystalline white solid (620 mg, 53%).
[0331] Characterization of the cocrystal:
[0332] The (+)-tramadol-(R)-naproxen (1:2) cocrystal obtained according to Example 2 passed 1 H-NMR, FTIR, powder X-ray diffraction, DSC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com