A kind of pharmaceutical composition and application for alleviating or eliminating opioid withdrawal syndrome
A composition and syndrome technology, applied in the fields of addiction medicine and neurobiology, can solve the problems of poor medication compliance and medication experience of patients, poor clinical application effect, and long withdrawal time, so as to improve medication compliance and Medication experience, obvious clinical advantages and, efficacy in eliminating withdrawal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0037] Embodiment 1-3 A compound tablet for alleviating or eliminating opioid withdrawal syndrome
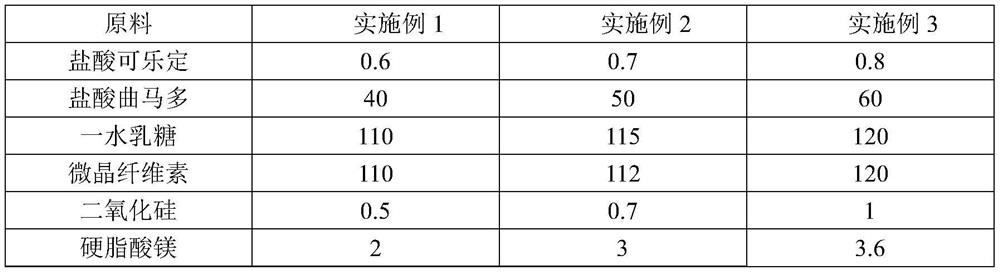
[0038] The prescription of a compound tablet for alleviating or eliminating opioid withdrawal syndrome according to Embodiment 1-3 is shown in Table 1.
[0039] Table 1 Prescription of a compound tablet for alleviating or eliminating opioid withdrawal syndrome (each tablet contains / mg)
[0040]
[0041]
Embodiment 4-10
[0042] Embodiment 4-10 A compound tablet for alleviating or eliminating opioid withdrawal syndrome
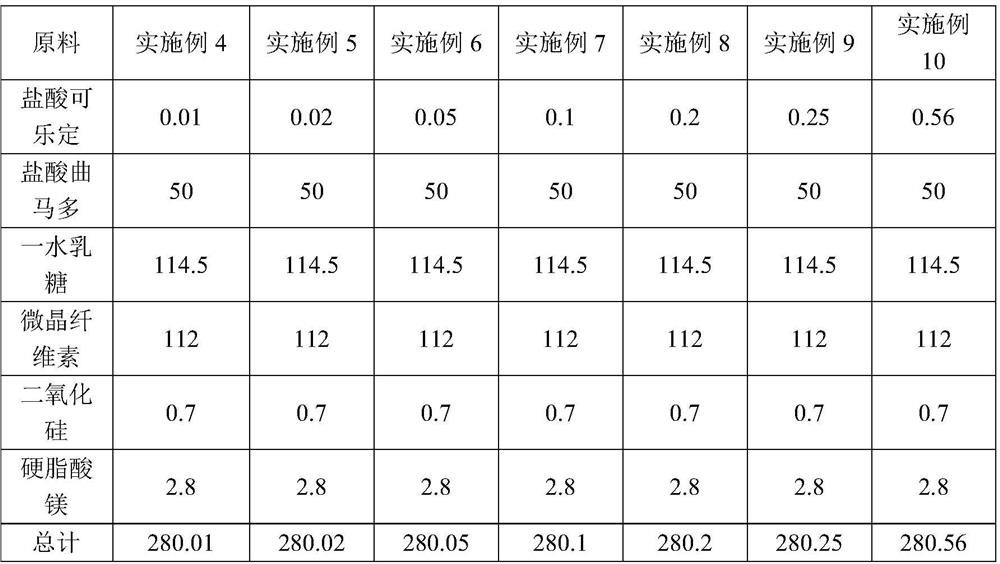
[0043] Table 2 shows the prescription of a compound tablet for alleviating or eliminating opioid withdrawal syndrome in Examples 4-10.
[0044] Table 2 Prescription of a compound tablet for alleviating or eliminating opioid withdrawal syndrome (each containing / mg)
[0045]
[0046] The preparation method of described compound tablet, comprises the following steps:
[0047] (1) dissolving the clonidine hydrochloride of the prescribed amount in water, sieving with the prescribed amount of microcrystalline cellulose after granulation, and drying to obtain granules;
[0048] (2) Mix the granules obtained in step (1) with the prescribed amount of tramadol hydrochloride and lactose monohydrate, then add the prescribed amount of silicon dioxide and magnesium stearate to mix, compress into tablets and coat, to obtain the obtained Description of compound tablets.
experiment example 2
[0086] Experimental Example 2. Main Pharmacodynamics Study on Inhibition of Morphine Withdrawal Symptoms by Drugs
[0087] (1) Mouse jumping test
[0088] Mice were randomly divided into blank control group and drug group 1-12 according to body weight, wherein, drug group 1-12 (respectively administer the compound tablet prepared by embodiment 1-10 and the single tablet prepared by comparative example 1-2), They were divided into low-dose group, middle-dose group and high-dose group, with 10 rats in each group. Each group was intraperitoneally injected with morphine, 6 times a day, the dosage was 10mg / kg / time on the first day, 10mg / kg / time on the 2nd-3rd day, 50 minutes after the last injection of morphine, the low dose group of the drug , middle-dose group and high-dose group were administered intragastrically at doses of 36mg / kg, 72mg / kg, and 143mg / kg, and the blank control group was given an equal volume of normal saline. After 30 minutes of intragastric administration, ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com