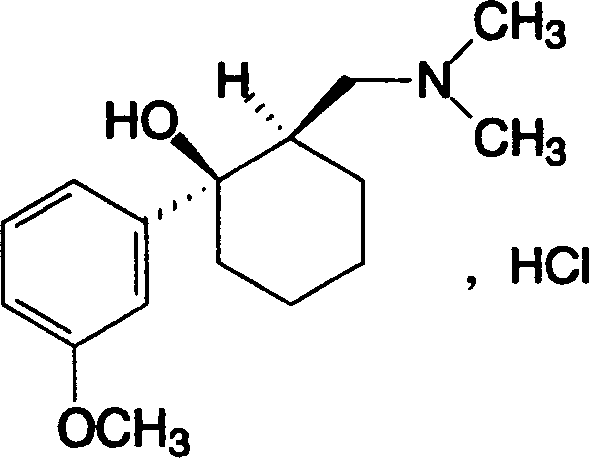
Tramadol hydrochloride oral disintegration tablets, and prepn. method therefor
A technology of tramadol hydrochloride and orally disintegrating tablets, which is applied in the field of tramadol hydrochloride orally disintegrating tablets and its preparation, can solve the problems of not fully meeting the needs of patients for medication and difficulty in taking medication, and achieve improved bioavailability, Good adhesion and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of Tramadol Hydrochloride Orally Disintegrating Tablets
[0045] components
weight
weight percentage
tramadol hydrochloride
L-HPC
50g
100 g
1 g
96 grams
12.6 grams
6 grams
2 grams
2.7 grams
0.4 grams
18.47%
36.94%
0.37%
35.46%
4.65%
2.22%
0.74%
1.00%
0.15%
Gross weight:
270.7 grams
[0046] Preparation Process:
[0047]Weigh tramadol hydrochloride, mannitol, sodium saccharin, citric acid, and part of microcrystalline cellulose according to the prescription amount, put them into a mixer and mix them evenly, add 50% ethanol solution as a wetting agent, stir for 5 minutes, and use 40 mesh Sieve granulation, ventilate and dry at 50°C±5°C, pass dry granules through a 40-mesh sieve f...
Embodiment 2
[0048] Embodiment 2: Preparation of Tramadol Hydrochloride Orally Disintegrating Tablets
[0049] components
weight
weight percentage
tramadol hydrochloride
50g
50g
1 g
85.4 grams
20 grams
9 grams
3 grams
2.2 grams
0.4 grams
22.62%
22.62%
0.45%
38.64%
9.05%
4.07%
1.36%
1.00%
0.18%
Gross weight:
221 grams
[0050] Preparation Process:
[0051] Weigh tramadol hydrochloride, erythritol, sodium saccharin, citric acid, and part of microcrystalline cellulose according to the prescription amount, put them into a mixer and mix them evenly, add an appropriate amount of 5% povidone, 95% ethanol solution, and stir for 5 minutes, use a 40-mesh sieve to granulate, ventilate and dry at 50°C±5...
Embodiment 3
[0052] Embodiment 3: Preparation of Tramadol Hydrochloride Orally Disintegrating Tablets
[0053] components
weight
weight percentage
tramadol hydrochloride
L-HPC
citric acid
50g
81.8 grams
2 grams
95.4 grams
10.6 grams
9 grams
3 grams
1 g
1 g
0.5 grams
19.66%
32.17%
0.79%
37.51%
4.17%
3.54%
1.18%
0.39%
0.39%
0.20%
Gross weight:
254.3 grams
[0054] Preparation Process:
[0055] Weigh tramadol hydrochloride, erythritol, sodium saccharin, citric acid, and part of microcrystalline cellulose according to the prescription, put them into a mixer and mix them evenly, add 50% ethanol solution as a wetting agent, stir for 5 minutes, and use Granulate with a 40-mesh sieve, ventilate and dry at 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com