Paliperidone extended-release mini pill
A technology of paliperidone and sustained-release pellets, which is applied in the directions of organic active ingredients, nervous system diseases, bulk transportation, etc., to achieve the effects of uniform particle size, cost reduction, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
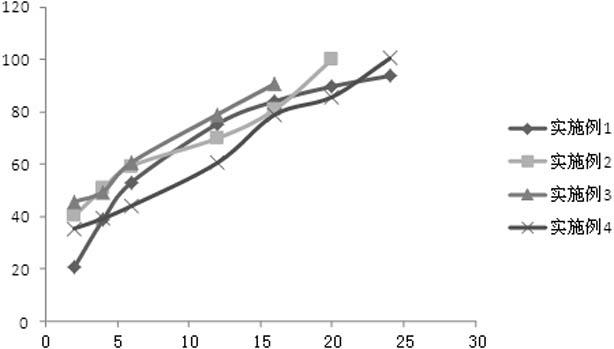
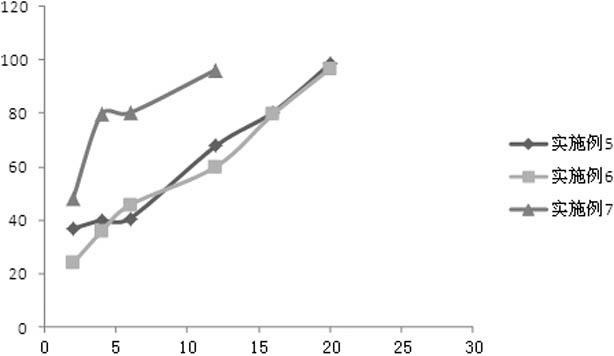
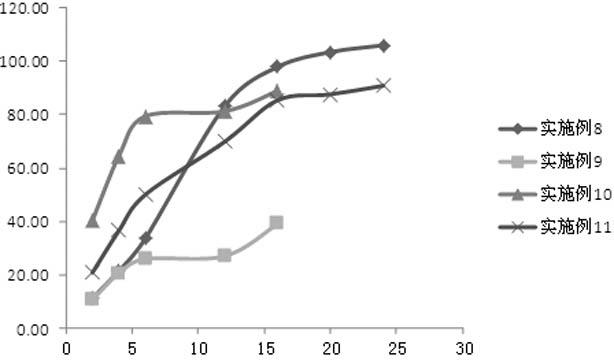
Embodiment 1
[0056] Preparation of paliperidone ordinary pellets: mix microcrystalline cellulose and paliperidone raw material according to the prescription ratio, add purified water to prepare wet material, and extrude through an extrusion sieve plate (aperture 1.0mm) to obtain The strip-shaped material is placed in a spheronizer and spheronized into pellets, dried in a tumbling cylinder, and sieved to sieve pellets with a diameter of 0.85-1.25 mm.
[0057] Preparation of coated pellets: Dilute the coating solution Surelease® with water to a certain concentration, add the prescribed amount of porogen, and stir evenly. Put the paliperidone ordinary pellets prepared above into the coating machine, after the temperature of the pellets rises to 35 ℃ ~ 40 ℃, slowly spray into the prepared coating solution for slow-release coating according to the prescription amount, and then dry have to.
Embodiment 2
[0059] Preparation method is the same as embodiment 1
Embodiment 3
[0061] Preparation method is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com