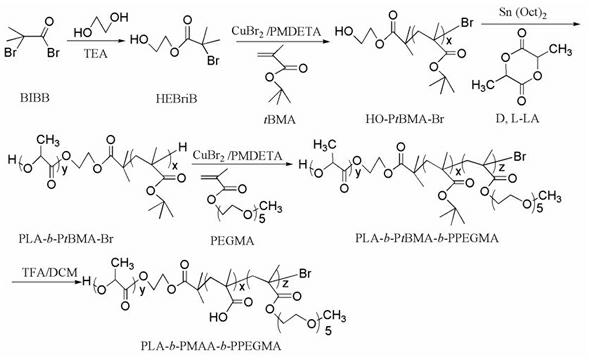
PH-responsive comb-like copolymer and preparation and application thereof
A comb-like copolymer and responsive technology, applied in the field of drug sustained and controlled release materials, can solve problems such as unsatisfactory performance, and achieve the effects of precise control of molecular weight and polydispersity, drug protection, and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
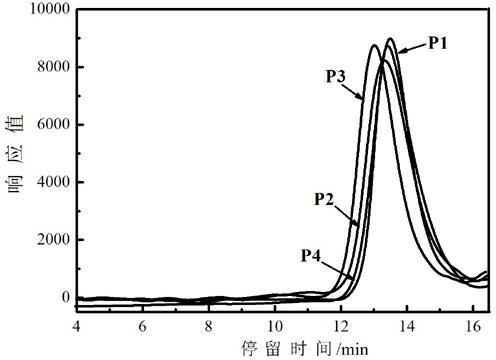
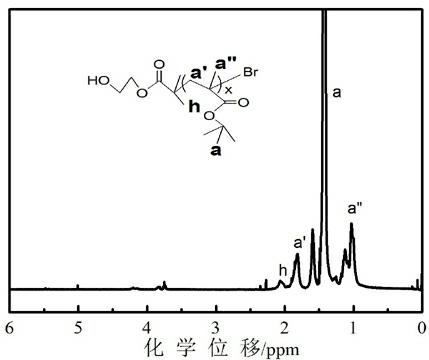
Image
Examples
Embodiment 1
[0075] Synthesis of the bifunctional initiator hydroxyethyl 2-bromoisobutyrate (HEBriB). Into a 150 mL reaction bottle, add a stirrer and seal it. After evacuating and blowing argon for 3 times, under the protection of argon, add ethylene glycol (112 ml, 2 mol), triethylamine (TEA, 11.13 ml, 80 mmol), cooled to 0 °C, and then slowly added 2-bromoisobutyryl bromide (0.989 ml, 80 mmol) dropwise, then reacted at 0 °C for 2 h, then raised the temperature to 40 °C for 5 h. After the reaction was completed, cool at room temperature, add 100 ml of deionized water, then extract 3 times with chloroform, combine the organic phases, and use dilute HCl, saturated NaHCO 3 The solution was washed with water and dried over anhydrous magnesium sulfate. Low boiling point substances were removed by rotary evaporation, and then HEBriB was distilled off under reduced pressure.
Embodiment 2
[0077] (1) Synthesis of macromolecular initiator HO-P t BMA-Br (target molecular weight 5000). Weigh a certain amount of CuBr 2 (11.2 mg, 0.05 mmol) was placed in a 50 mL dry eggplant-shaped bottle with a stirring bar, sealed with a reverse rubber stopper, and vacuum-filled with argon three times. Under the protection of argon, the solvent toluene (20 ml), the monomer t Add BMA (5.715 mL, 35.2 mmol) and ligand PMDETA (0.210 mL, 1 mmol) into the bottle, stir for 15 min to form the catalyst complex, add the reducing agent Sn(Oct) 2(0.162 mL, 0.5 mmol) and then stirred for 5 min. After three freezing-pumping-heating cycles with liquid nitrogen, argon was introduced, and the initiator HEBriB (0.165 mL, 1 mmol) was added dropwise with a syringe, and then transferred to an oil bath at 90 °C for 2 h. After the reaction is complete, cool to room temperature, add 50 mLTHF, stir to dissolve, and then use a neutral alumina column to remove the catalyst CuBr 2 (THF as eluent). The o...
Embodiment 3
[0083] (1) Synthesis of macromolecular initiator HO-P t BMA-Br (target molecular weight 8400). Weigh a certain amount of CuBr 2 (11.2 mg, 0.05 mmol) was placed in a 50 mL dry eggplant-shaped bottle with a stirring bar, sealed with a reverse rubber stopper, and vacuum-filled with argon three times. Under the protection of argon, the solvent toluene (20 ml), the monomer t Add BMA (6.751 mL, 59 mmol) and ligand bpy (0.134 mL, 1 mmol) into the bottle, stir for 15 min to form the catalyst complex, add the reducing agent Sn(Oct) 2 (0.224 mL, 0.7 mmol) and then stirred for 5 min. After three freezing-pumping-heating cycles with liquid nitrogen, argon was introduced, and the initiator HEBriB (0.214 mL, 1.33 mmol) was added dropwise with a syringe, and then transferred to an oil bath at 60 °C for 4 h. After the reaction is complete, cool to room temperature, add 50 mLTHF, stir to dissolve, and then use a neutral alumina column to remove the catalyst CuBr 2 (THF as eluent). The ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com