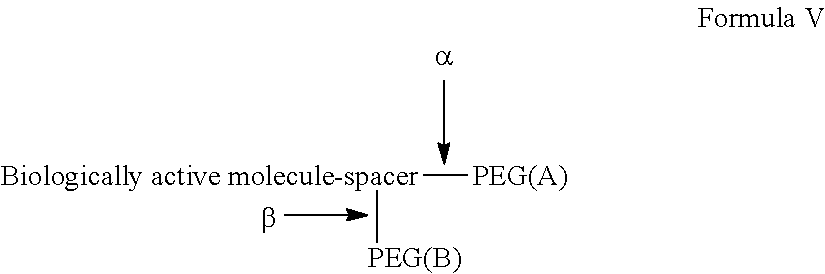
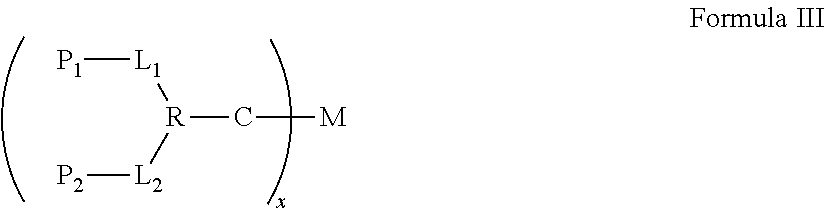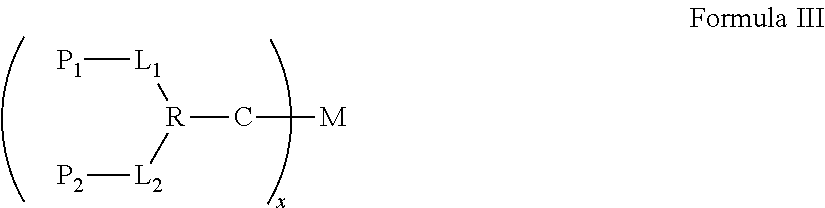Synergistic biomolecule-polymer conjugates
a biomolecule and polymer technology, applied in the field of synergistic biomolecule polymer conjugates, can solve the problems of reducing the bioactivity of the parent protein and organic therapeutic molecules, reducing the biological activity of the proteins that are pegylated by conventional permanent branched or linear peg compounds, etc., to achieve the effect of exacerbate systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fmoc-Lys(Boc)-20K mPEG Ester 1
[0108]To a solution of 20 kDa mPEG (1 g, 0.05 mmol) and Fmoc-Lys(Boc)-OH (117mg, 0.25 mmol) in anhydrous dichloromethane cooled in an ice-water bath was added dicyclohexylcarbodiimide (166 mg, 0.8 mmol), and the mixture was stirred under nitrogen and allowed to warm to room temperature overnight. The N,N′-dicyclohexylurea was removed from the reaction mixture by filtration. The filtrate was dried in vacuo. The residue was dissolved in anhydrous dichloromethane, and the white solid was precipitated by addition of tert-butyl methyl ether. The white solid product 1 was collected and washed with tert-butyl methyl ether.
example 2
Fmoc-Lys(α-10K mPEG, carbamate)-20K mPEG Ester 3
[0109]To a solution of Fmoc-Lys(Boc)-20K mPEG Ester 1 (0.8 g, 0.04 mmol) in anhydrous dichloromethane (4 mL) was added 4 mL of Trifluoroacetic acid. The reaction was stirred for one hour at room temperature, and the white solid was precipitated by tert-butyl methyl ether (70 mL). The solid product Fmoc-Lys-20K mPEG Ester 2 was collected and washed by tert-butyl methyl ether.
[0110]A solution Fmoc-Lys-20K mPEG Ester 2 (0.35 g, 0.018 mmol), 10K mPEG succinimidyl carbonate (SC-mPEG) (225 mg, 0.022 mmol), and triethylamine (110 mg, 1.08 mmol) in anhydrous methylene chloride (9 mL) was stirred at room temperature under nitrogen overnight. The reaction mixture was concentrated by partial removal of solvent under vacuum. The product Fmoc-Lys(α-10K mPEG, carbamate)-20K mPEG Ester 3 was precipitated by addition of tert-butyl methyl ether, filtered and collected. Product 3 can be further purified by chromatography.
example 3
30k Da Lys (α-10kPEG carbamate, 20kPEG ester) succinimidyl suberate ester 5
[0111]Fmoc-Lys(α-10K mPEG, carbamate)-20K mPEG Ester 3 (0.5 g, 0.016 mmol) was dissolved in 11 mL of a mixture of dichloromethane / diethylamine (5:6), and the reaction mixture was stirred at room temperature for 3.5 hours. The solid product 4 was precipitated by addition of tert-butyl methyl ether (70 mL), filtered, and collected.
[0112]A solution of Lys(α-10K mPEG, carbamate)-20K mPEG Ester 4 (0.4 g, 0.013 mmol), Suberic acid bis(N-hydroxysuccinimide ester) (26 mg, 0.07 mmol) and triethylamine (3 mg, 0.03 mmol) in a mixture of anhydrous methylene chloride (7 mL) and dimethylformamide (3 mL) was stirred at room temperature under nitrogen for 9 hours. The reaction mixture was concentrated by partial removal of solvent under vacuum, and the white solid was precipitated by addition of tert-butyl methyl ether. The solid product 5 was collected and washed by tert-butyl methyl ether.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com