Osmotic pump drug delivery systems and methods
a technology of osmotic pump and drug delivery system, which is applied in the direction of flow control, medical devices, instruments, etc., can solve the problems of infection such a significant risk, unsatisfactory side effects, and often limited iv administration to a number of weeks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
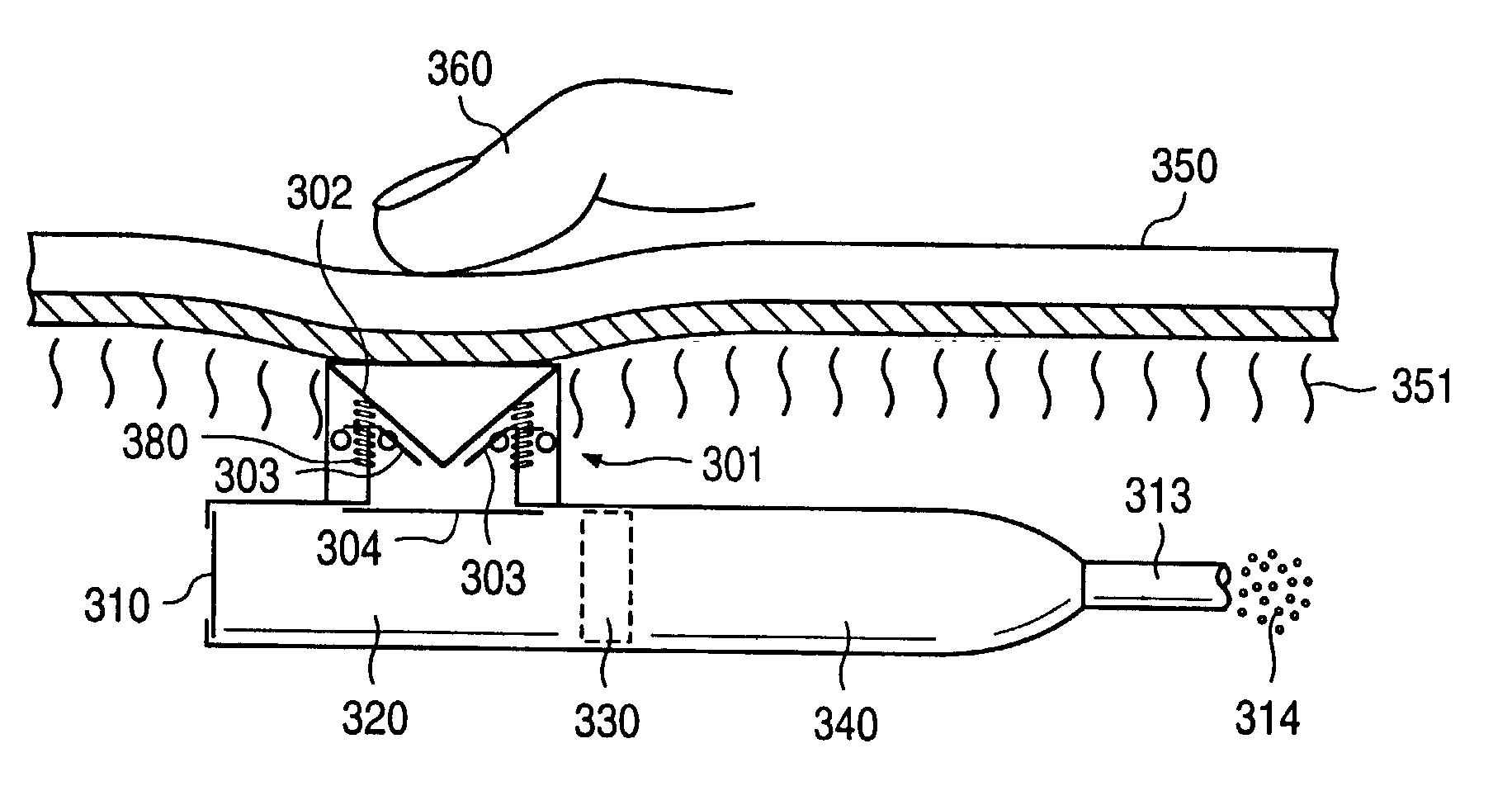
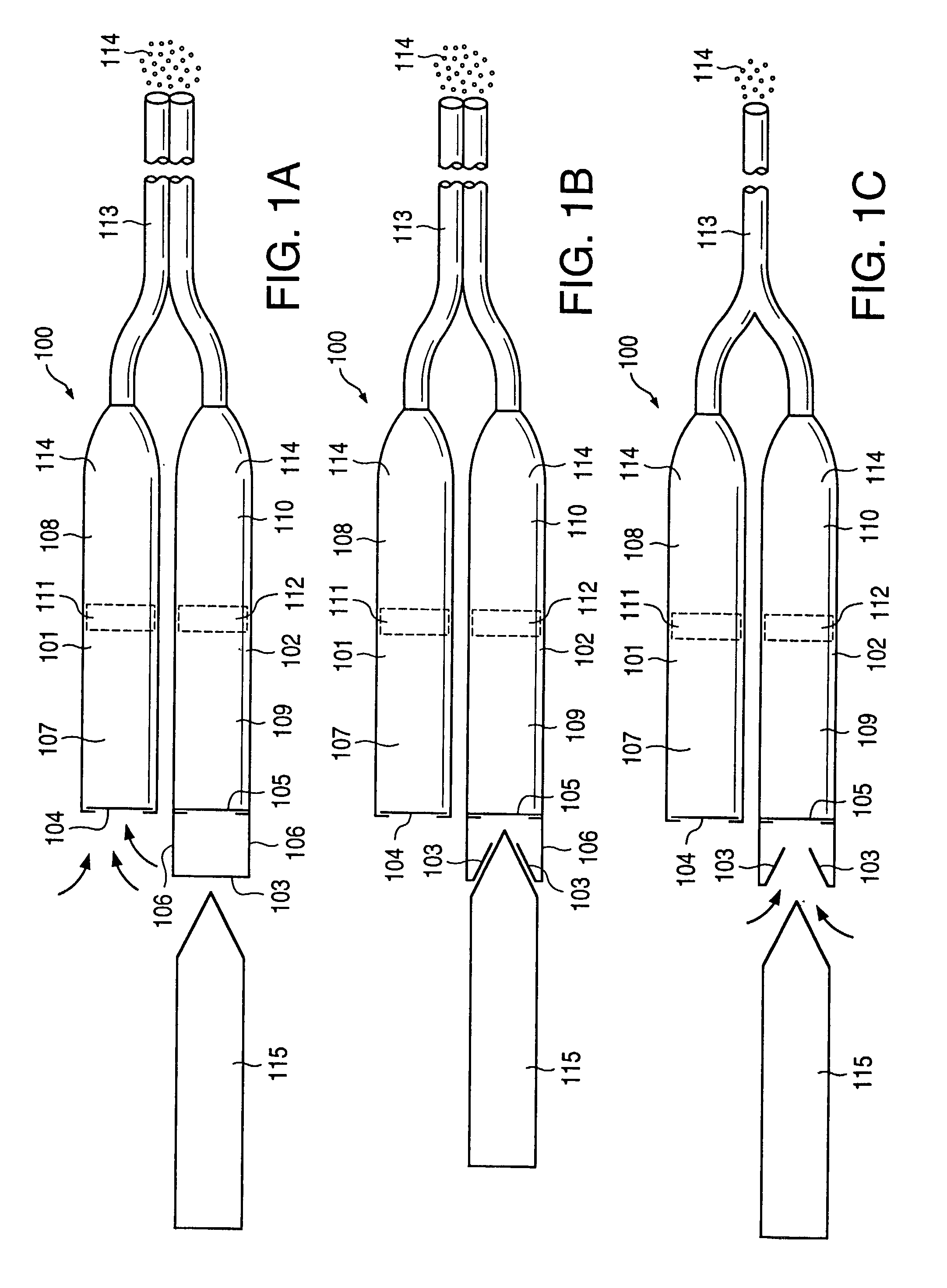
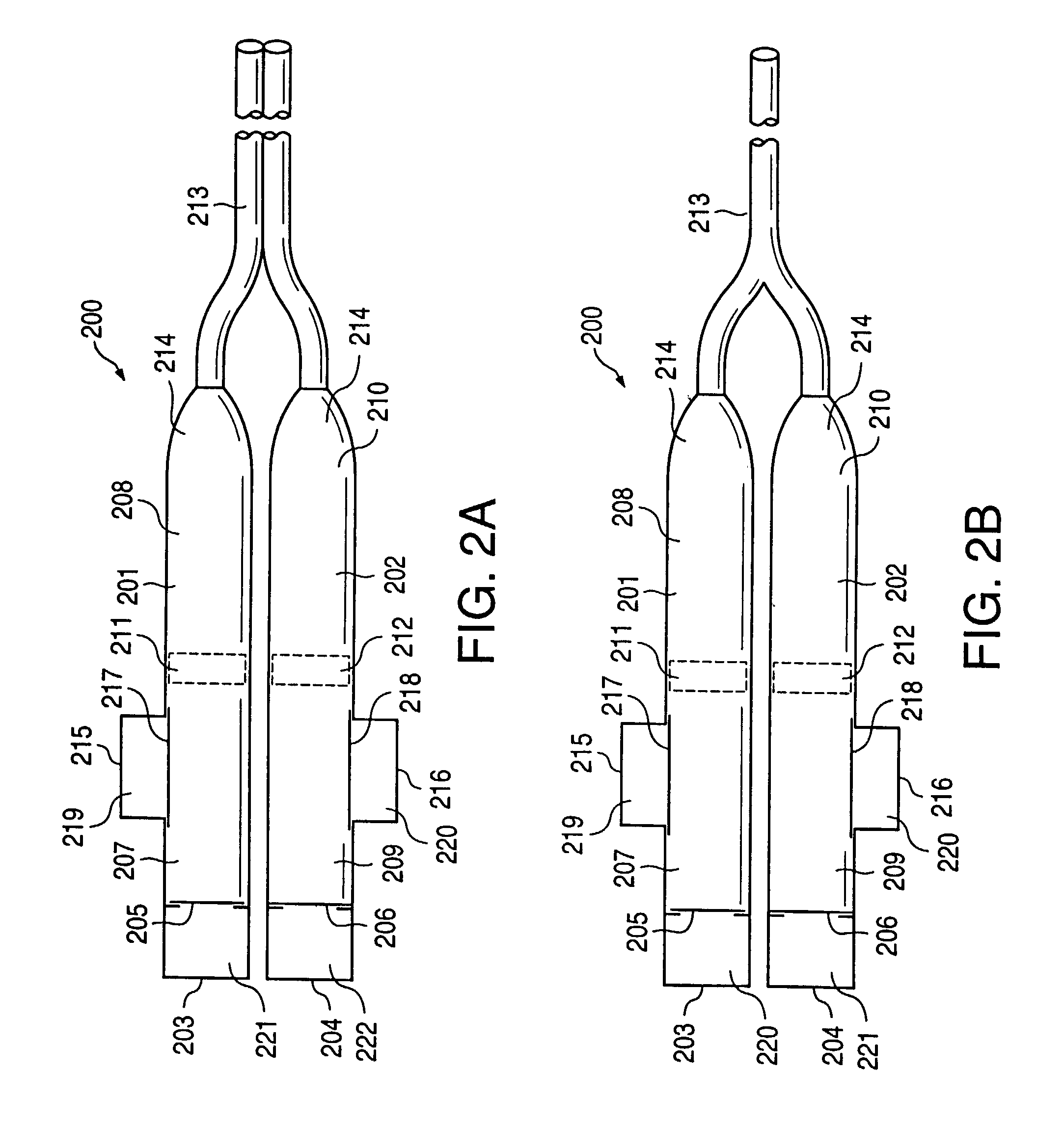
[0043] FIG. 11a illustrates an implantable osmotic delivery system, kit and method according to an embodiment of the present invention. As shown therein, the osmotic pump system 100 according to the present invention includes a first pump 101 and a second pump 102. The first and pump 101 includes an osmotic engine compartment 107 and a pharmaceutical agent compartment 108 that are separated from one another by a movable piston 111 (shown in dashed lines). Similarly, the second implantable osmotic pump 102 includes an osmotic engine compartment 109 and a pharmaceutical agent compartment 110 that are separated from one another by a movable piston 112 (also shown in dashed lines). Each of the osmotic pumps 101 and 102 has a proximal and a distal end. A catheter 113 is attached to the distal end of each of the first and second pumps 101, 102. The catheter 113 may be a dual lumen catheter as shown in FIGS. 1a and 1b and include a first lumen and a second lumen, the first lumen being conn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com