Antifungal medicament solid dispersion
A solid dispersion, posaconazole solid technology, applied in antifungal agents, pharmaceutical formulations, organic active ingredients and other directions, can solve problems such as low bioavailability, and achieve the effects of increasing solubility, improving stability and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
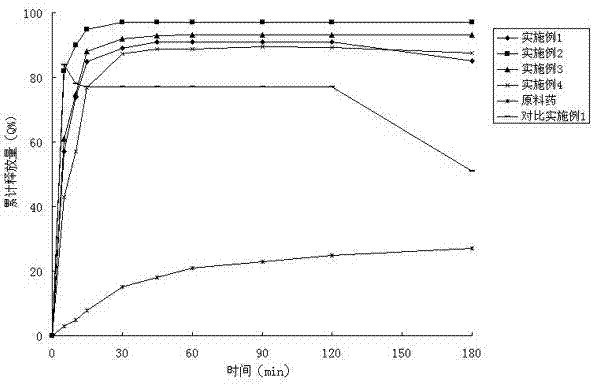
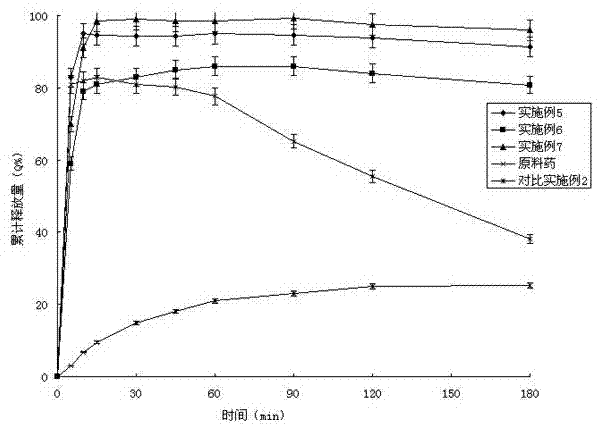
Embodiment 1
[0037] Example 1 Preparation of solid dispersions containing posaconazole and PVPVA64 with Eudragit E100
[0038] Co-rotating twin-screw extruder TE-20 (Coperon Keya, Germany) was used to set the temperature from each section to the head, and after 20 minutes of equilibrium, the screw speed was set to 25r / min, and posaconazole, Put 200g of the physical mixture of PVPVA64 and Eudragit E100 into the hopper with a mass ratio of 100:20:80. After 1 minute, the material die hole is extruded in strips, and the extrudate is placed on an aluminum plate and cooled to room temperature. After standing under the condition for 4 hours, crush and pass through 80-mesh sieve to obtain white powder.
Embodiment 2
[0039] Example 2 Preparation of solid dispersion containing posaconazole and Soluplus? with Eudragit E100
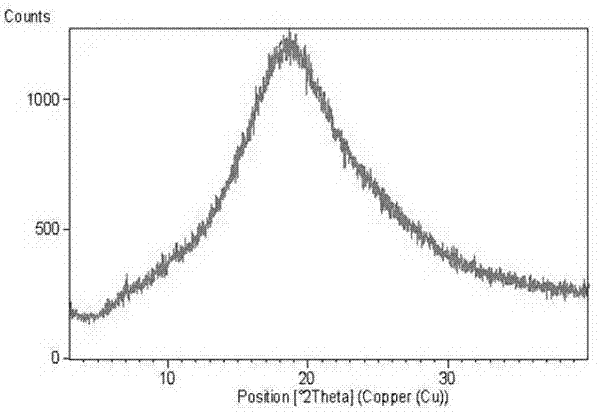
[0040] Co-rotating twin-screw extruder TE-20 (Coperon Keya, Germany) was used to set the temperature from each section to the head, and after 20 minutes of equilibrium, the screw speed was set to 25r / min, and posaconazole, Put 200g of Soluplus? and Eudragit E100 into the hopper with a mass ratio of 80:48:72. After 1 minute, the material die hole is extruded in strips, and the extrudate is placed on an aluminum plate and cooled to After standing at room temperature for 4 hours, pulverize and pass through an 80-mesh sieve to obtain a white powder, the X-ray powder diffraction pattern is shown in the attached figure 1 .
Embodiment 3
[0041] Example 3 Preparation of solid dispersion containing posaconazole and Soluplus? with HPMC
[0042] Co-rotating twin-screw extruder TE-20 (Coperon Keya, Germany) was used to set the temperature from each section to the head, and after 20 minutes of equilibrium, the screw speed was set to 25r / min, and posaconazole, Put 200g of the physical mixture of Soluplus® and HPMC into the hopper with a mass ratio of 33.3:33.3:133.4. After 1min, the material die hole is extruded in strips, and the extrudate is placed on an aluminum plate and cooled to room temperature. After standing under the condition for 4 hours, crush and pass through 80-mesh sieve to obtain white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com