Sulfide-based lithium-ion-conducting solid electrolyte glass, all-solid lithium secondary battery, and method for manufacturing all-solid lithium secondary battery
A solid electrolyte, lithium secondary battery technology, used in secondary batteries, battery electrodes, non-metallic conductors, etc., can solve problems such as low operating voltage, avoid electrical short circuits, high industrial value, and excellent charge-discharge cycle life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
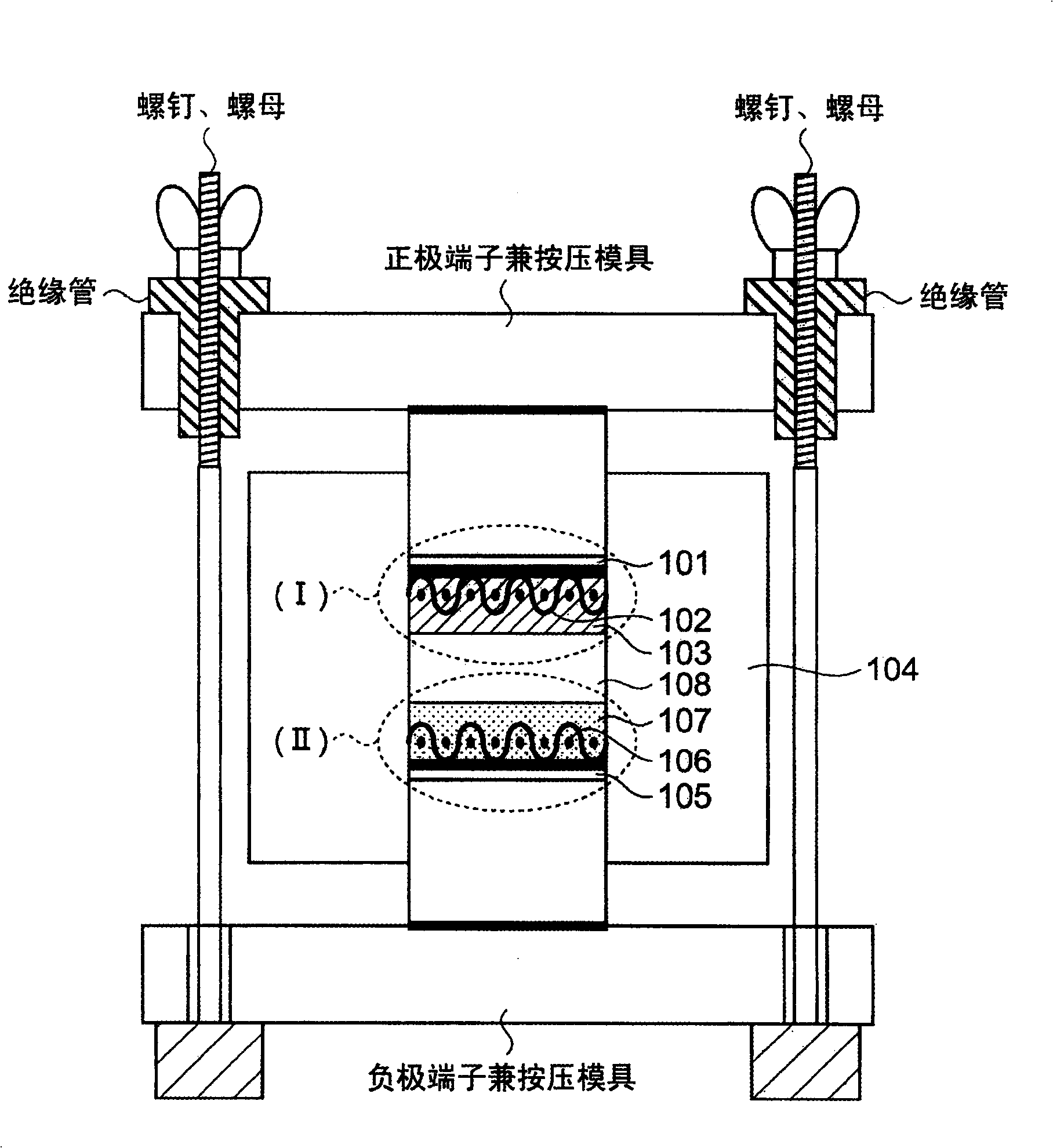
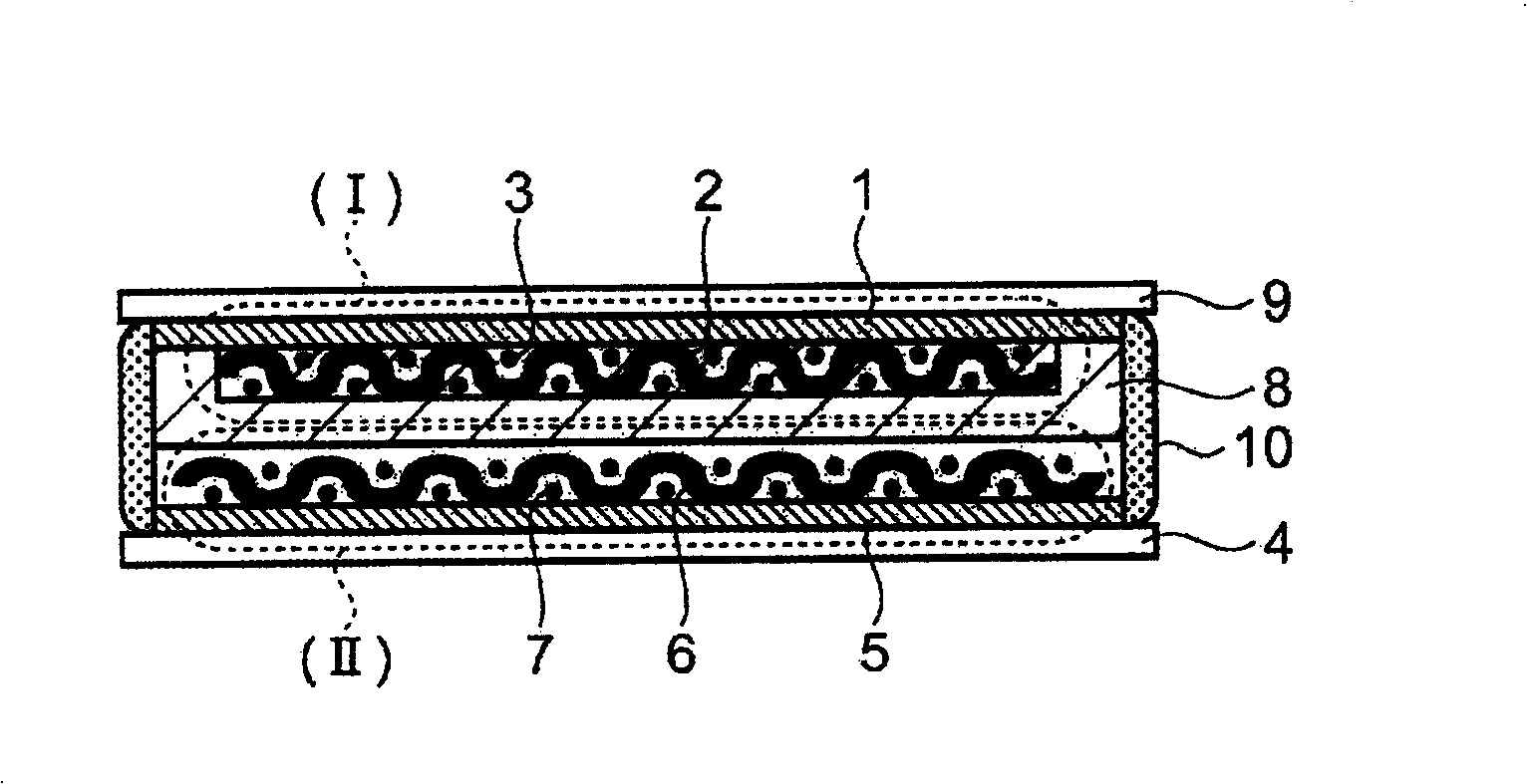
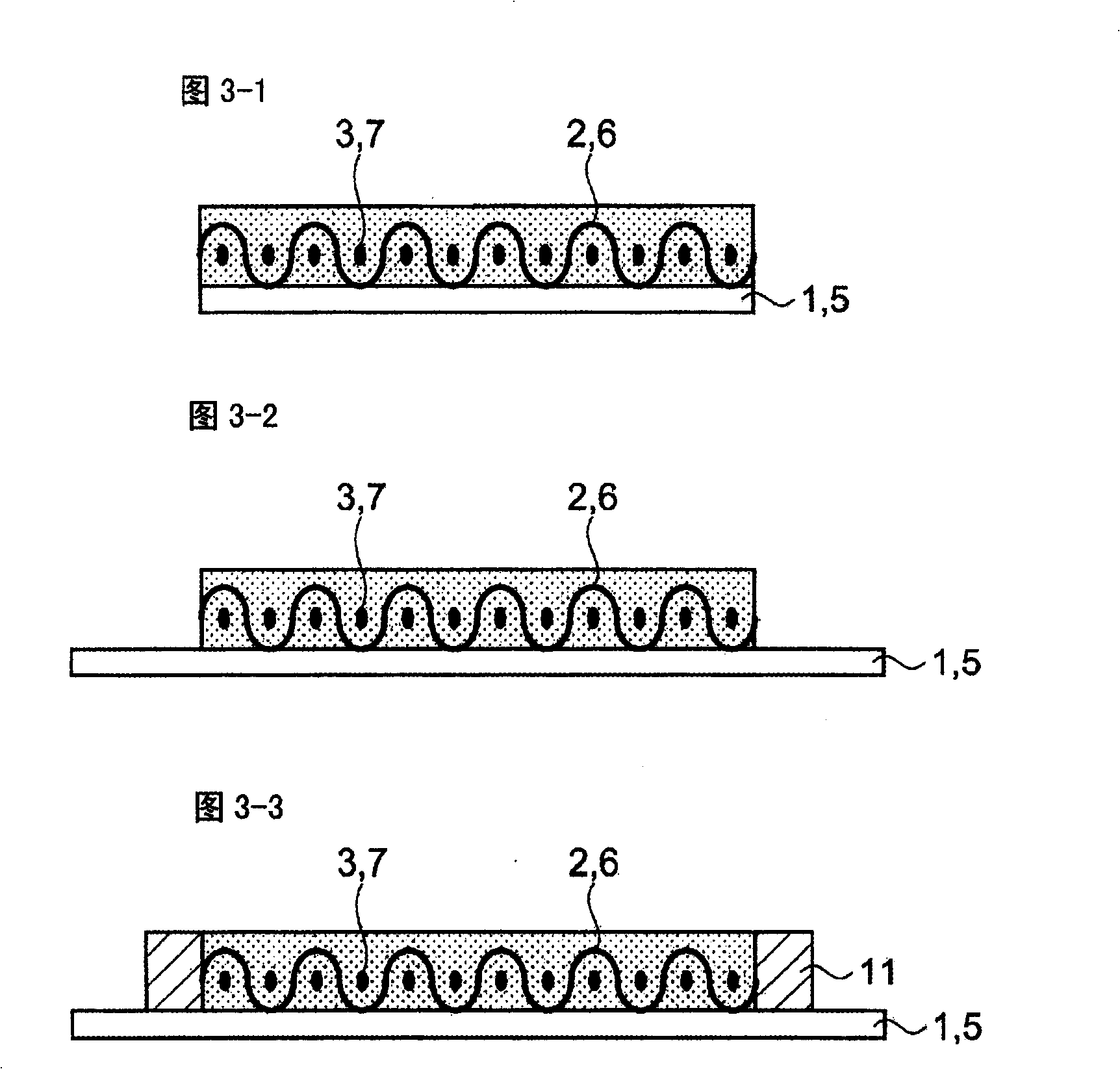
[0074] The sulfide-based lithium ion conductive solid electrolyte used in Embodiment 1 is a glassy electrolyte formed by containing α-alumina in the sulfide-based lithium ion conductive solid electrolyte. For example Li 2 S-SiS 2 , Li 2 S-SiS 2 -LiI, Li 2 S-SiS 2 -LiBr, Li 2 S-SiS 2 -LiCl, Li 2 S-SiS 2 -B 2 S 3 -LiI, Li 2 S-SiS 2 -P 2 S 5 -LiI, Li 2 S-B 2 S 3 , Li 2 S-B 2 S 3 -LiI, Li 2 S-P 2 S 5 , Li 2 S-P 2 S 5 -LiI, Li 2 S-P 2 S 5 -ZmSn (Z=Ge, Zn, Ga), Li 2 S-GeS 2 , Li 2 S-SiS 2 -Li 3 PO 4 , Li 2 S-SiS 2 -LixPOy (M=P, Si, Ge, B, Al, Ga, In)-based sulfide lithium ion conductive solid electrolyte glass and crystalline lithium ion conductors containing these components or lithium ions formed from mixtures thereof Conductive solid electrolyte.
[0075] Next, α-alumina having a particle size of 10 μm or less is mixed and used as insulating fine particles in these sulfide-based lithium ion conductive solid electrolytes as a matrix. The α-al...
Embodiment 1
[0084] Here, by Li 2 S-SiS 2 -Li 3 PO 4 The formed lithium ion conductive glass was used as a starting material, that is, a sulfide-based lithium ion conductor, and a new glass obtained by mixing α-alumina as insulating fine particles at a weight ratio of 7% was formed by the method described in 1) above. Sulfide-based lithium ion conductive solid electrolyte glass.
[0085] The obtained sulfide-based lithium-ion conductive solid electrolyte glass was pulverized with a planetary ball mill to an average particle size of about 7 microns, and the obtained solid electrolyte powder was filled into a cylinder having a diameter of 1 cm and having an ion conductivity measurement unit. Forming fixture made of alumina, at 2 tons / cm 2 Formed under high pressure. In this pressurization, press molding is performed while heating the jig to the softening temperature range (approximately 200°C to 320°C) of the sulfide-based lithium ion conductive solid electrolyte glass (heating time is ...
Embodiment 2
[0091] Here, in order to analyze the relationship between the heating of the electrolyte glass used in Example 1 and the heating temperature and time in compression molding, an electrolyte glass molded body was produced in the same manner, its conductivity was measured, and its appearance was observed. However, the molding pressure used here is the same as in Example 1, which is 2 tons / cm 2 . The obtained results are combined and shown in Figure 11 . From this result, it can be seen that when the heating temperature is heated and compressed in the temperature range of 180° C. to 350° C., if the treatment time is within 6 hours, the ion conductivity of all glass molded objects is 1×10 -3 S / cm 2 above. However, when the treatment temperature is 300° C., if the treatment time exceeds 5 hours, the ion conductivity slightly decreases in this temperature range. It can be seen that the treatment temperature is preferably in the temperature range of 200°C to 300°C, and the treat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com