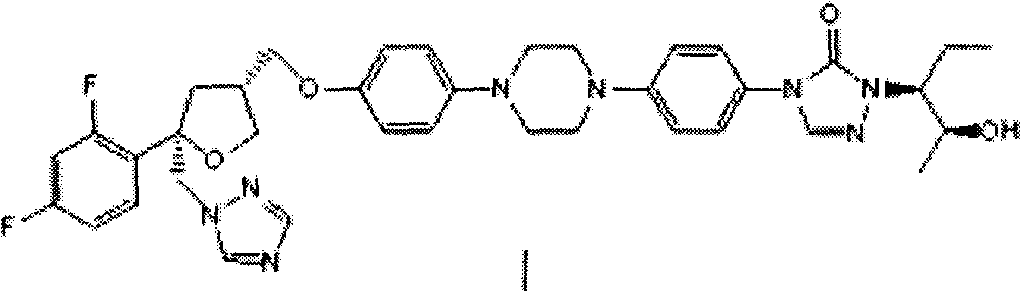
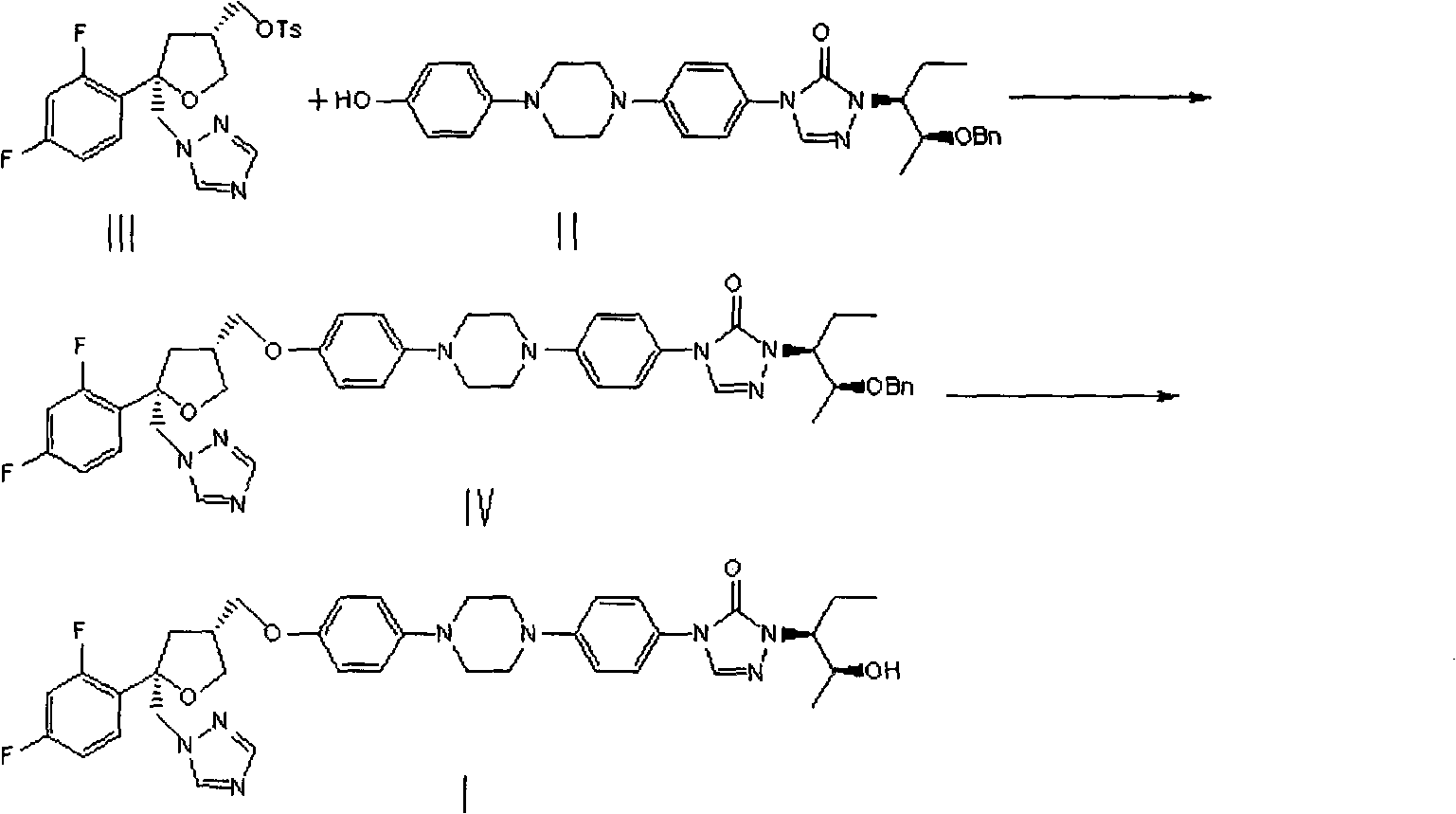
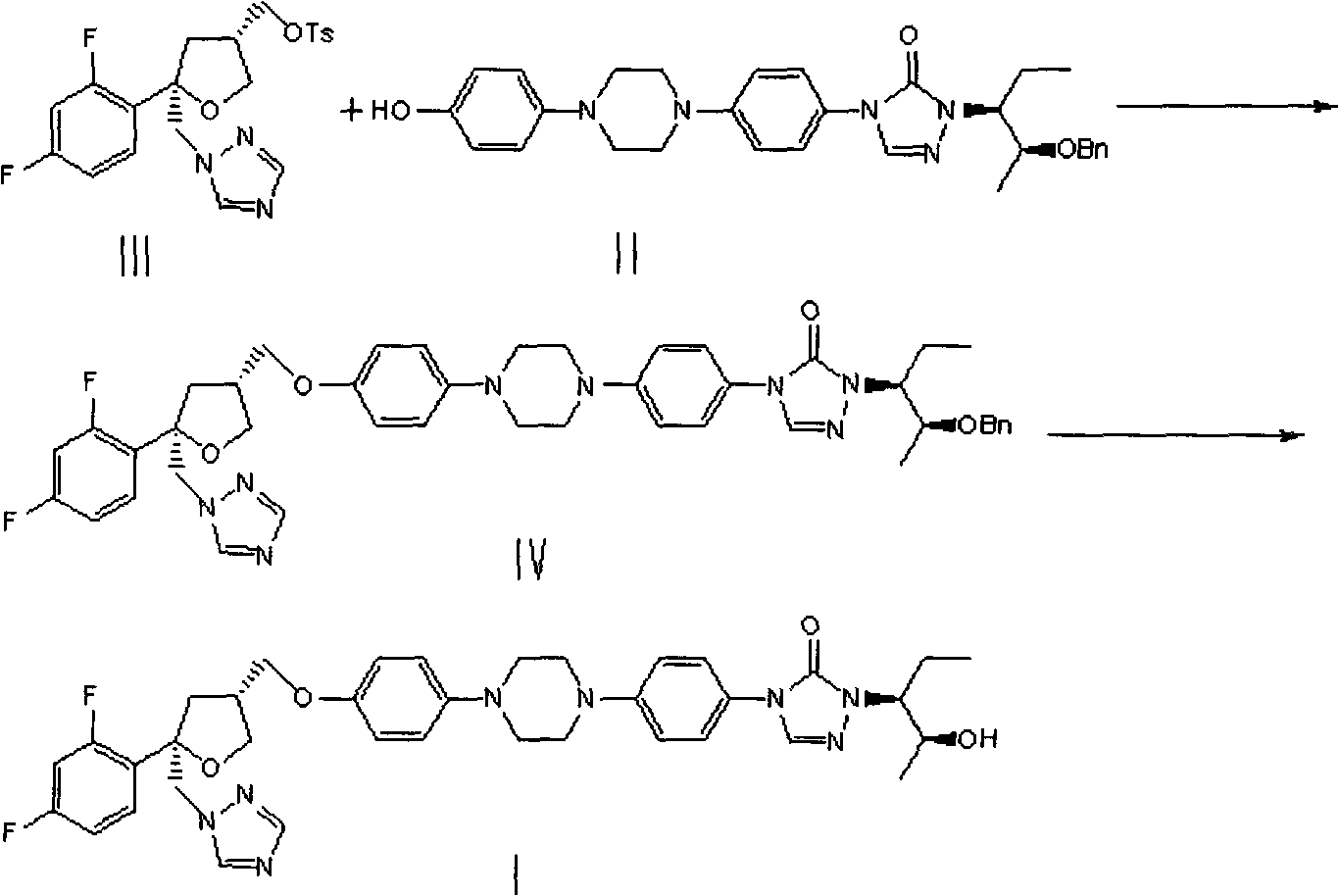
Preparation method of posaconazole
A technology for posaconazole and a compound, which is applied in the field of preparation of posaconazole, can solve the problems of dangerous technical operation, unsuitability for industrialized production, and high production cost, and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) Oxolan-3-yl"methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-benzyloxypent-3-yl]-1, 2, the preparation of 4-triazol-3-ketone (IV):
[0028] Add (1S)-(2S)-2-(2-benzyloxy-1-ethyl-propyl)-4-{4-[4-(4-hydroxy-phenyl)-piperidine to a 100mL three-necked flask Oxyzin-1-yl]-phenyl]-2,4-dihydro-1,2,4-triazol-3-one (II) (4.78 g, 9.31 mmol) and DMSO (40 mL), stirred and dissolved. Then 25% aqueous NaOH (1.6 mL, 9.4 mmol) was added and the mixture was stirred for 10 min. Add (5R,3R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazole-1-methyl)tetrahydrofuran-3-methanol p-toluenesulfonate (III) (4.40g, 9.79mmol), the mixture was reacted at 30°C for about 12-14h. The liquid phase was monitored for the completion of the reaction, and the reaction solution was slowly poured into vigorously stirred water (240 mL). After the addition was complete, the mixture was stirred vigorously for 10 minutes, filtered, the filter cak...
Embodiment 2
[0032] 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) Oxolan-3-yl"methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-benzyloxypent-3-yl]-1, 2, the preparation of 4-triazol-3-ketone (IV):
[0033] Add (1S)-(2S)-2-(2-benzyloxy-1-ethyl-propyl)-4-{4-[4-(4-hydroxy-phenyl)-piperidine to a 100mL three-necked flask Azin-1-yl]-phenyl 1-2,4-dihydro-1,2,4-triazol-3-one (II) (5.02g, 9.77mmol) and DMF (40mL), stirred and dissolved. Then 2.0 ml of 25% aqueous NaOH solution was added, and the mixture was stirred for 10 minutes. Add (5R,3R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazole-1-methyl)tetrahydrofuran-3-methanol p-toluenesulfonate (III) (6.25g, 13.91mmol), the mixture was reacted at 35°C for 12-14h. The liquid phase was monitored for the completion of the reaction, and the reaction solution was slowly poured into vigorously stirred water (240 mL). After the addition was complete, the mixture was stirred vigorously for 10 minutes, filtered, the filter cake was...
Embodiment 3
[0037] 4-[4-[4-[4-[[(3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) Oxolan-3-yl"methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-benzyloxypent-3-yl]-1, 2, the preparation of 4-triazol-3-ketone (IV):
[0038] Add (1S)-(2S)-2-(2-benzyloxy-1-ethyl-propyl)-4-{4-[4-(4-hydroxy-phenyl)-piperidine to a 100mL three-necked flask Oxyzin-1-yl]-phenyl]-2,4-dihydro-1,2,4-triazol-3-one (II) (5.37g, 10.46mmol) and DMF (40mL), stirred and dissolved. Then 25% aqueous NaOH (2.1 mL) was added and the mixture was stirred for 10 minutes. Add (5R,3R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazole-1-methyl)tetrahydrofuran-3-methanol p-toluenesulfonate (III) (3.76g, 0.84mmol), the mixture was reacted at 20°C for 12-14h. The liquid phase was monitored for the completion of the reaction, and the reaction solution was slowly poured into vigorously stirred water (240 mL). After the addition was complete, the mixture was stirred vigorously for 10 minutes, filtered, the filter cake was washed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com