Posaconazole solid dispersion and preparation method thereof
A technology of posaconazole solid and posaconazole, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of low dissolution rate, poor solubility, and low bioavailability, etc. Achieve the effects of continuous operation, stable preparation quality and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Posaconazole and Soluplus Solid Dispersions by Hot Melt Extrusion
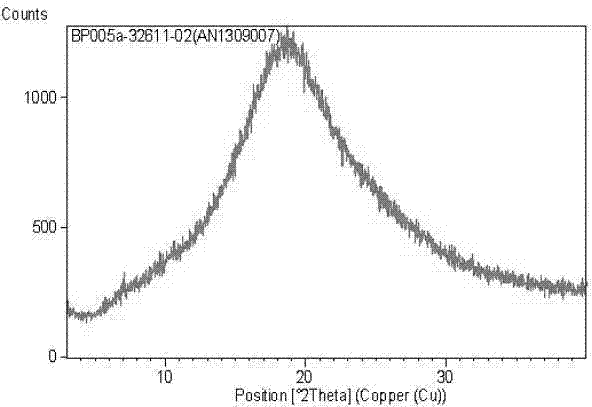
[0034] Co-rotating twin-screw extruder TE-20 (Coperon Keya, Germany) was used to set the temperature from each section to the head. After 20 minutes of equilibrium, the screw speed was set to 25r / min, and posaconazole and Put 200g of the physical mixture of Soluplus 1:6 (mass ratio) into the hopper. After 1min, the die hole of the material machine head is extruded in strips, and the extrudate is placed on a glass plate, cooled to room temperature and placed for 4 hours. , pulverized, and crossed an 80-mesh sieve to obtain a white powder. X-ray powder diffraction showed that posaconazole was dispersed in Soluplus in an amorphous state (as attached figure 1 shown).
Embodiment 2
[0035] Example 2 Preparation of solid dispersions of posaconazole and Soluplus by melting method
[0036] 33 grams of Soluplus and 67 grams of posaconazole are blended together until a uniform blend of posaconazole and Soluplus is made, then heated and melted, stirred, and after posaconazole is fully dispersed in the carrier, placed Stir vigorously on an ice-salt bath, cool rapidly to solidify, then place in a -20°C refrigerator for 2 hours, dry in a desiccator for 2 days, crush, and pass through an 80-mesh sieve for later use.
Embodiment 3
[0037] Example 3 Preparation of solid dispersions of posaconazole and Soluplus by freeze-drying
[0038] Dissolve 10g of posaconazole in a mixed solution of 50ml of methanol and concentrated hydrochloric acid (methanol:concentrated hydrochloric acid=10:1, V / V), stir and mix evenly; dissolve 100g of Soluplus in purified water to prepare a 30% aqueous solution; The above two solutions were mixed and stirred for 2 h to obtain a clear solution; the above clear solution was filtered through a 0.45 μm polytetrafluoroethylene filter (Rezist 30, Whatman, Dassel, Germany); cooled with liquid nitrogen, and finally lyophilized for 56 h to obtain a white solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com