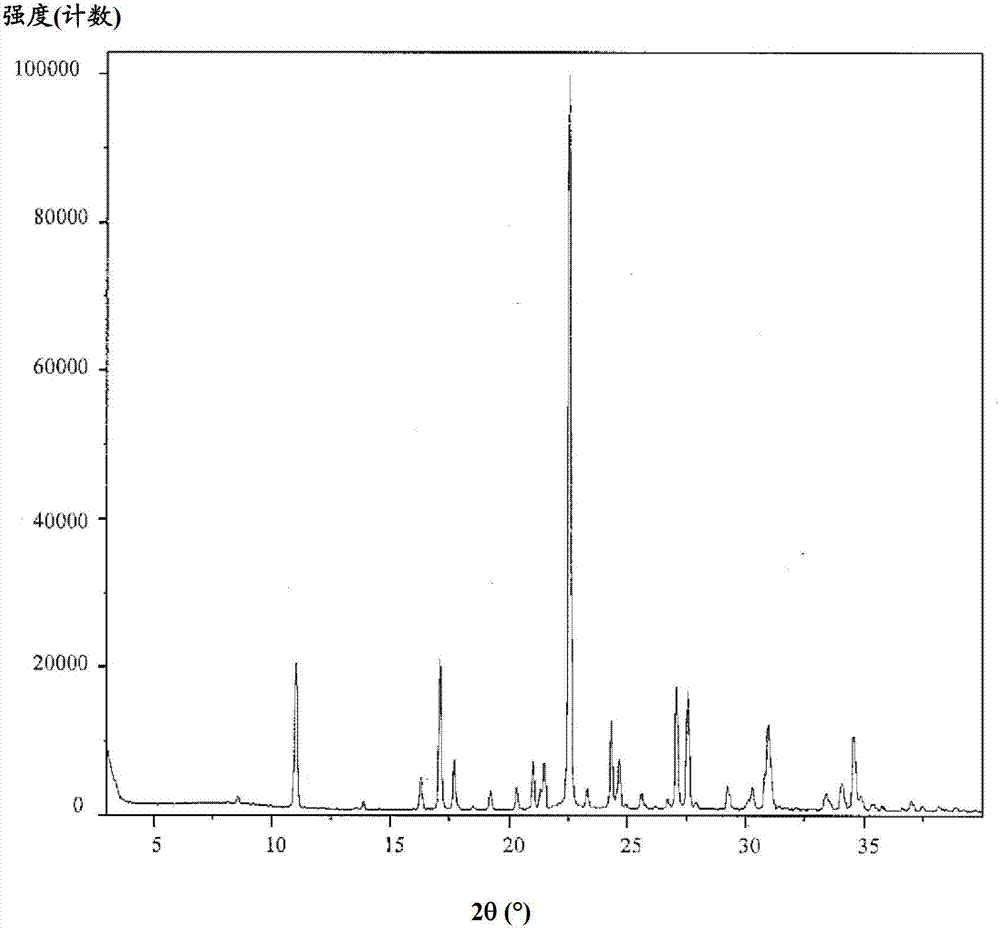
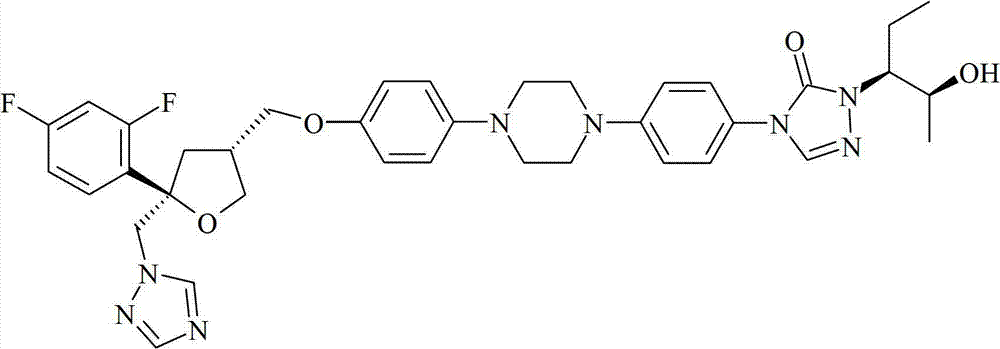
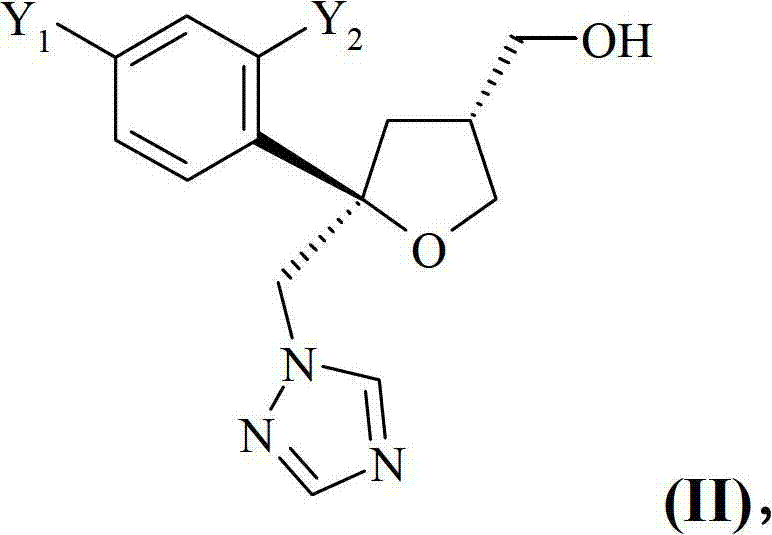
Purification of posaconazole and posaconazole intermediates
A technology of compounds and trans isomers, applied in the field of purification of chiral compounds, can solve problems such as undesired starting points
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0139] According to another preferred embodiment of the present invention, the compound of formula (C) obtained by step (ii) is suitably reduced to obtain the compound of formula (D):
[0140]
[0141]The reduction in step (iii) can be carried out according to any suitable method involving any suitable reducing agent. According to the present invention, hydride reducing agents are preferably used. Such a hydride reducing agent is, for example, sodium borohydride (NaBH 4 ), lithium borohydride (LiBH) 4 ), lithium aluminum hydride (LiAlH 4 ), diisobutylaluminum hydride (DIBAL) or lithium triethylborohydride (LiEt 3 BH). According to a preferred embodiment of the present invention, LiBH is used in step (iii) 4 as a reducing agent.
[0142] According to the prior art, at least 3 molar equivalents of LiBH have to be used relative to the compound of formula (C) 4 . See WO 94 / 25452, page 31, section "Preparation 5". Contrary to the teachings of the prior art, however, the...
Embodiment 1
[0291] Example 1: Preparation of compounds of formula (I)
[0292] (a) Preparation of compound of formula (Ba)
[0293] 3.8g Mg was suspended in 20ml MTBE. The temperature of the suspension was 55°C. Then, 0.5 g of Grignard reagent (CH 3 ) 3 Si-CH 2 MgCl was added to dry the system (if this Grignard reagent was not available in the first batch, (CH in ether) commercially available from Sigma-Aldrich as a 1.0 M solution can be used. 3 ) 3 Si-CH 2 MgCl (CAS Reg. No.: 13170-43-9)), followed by the addition of 1.0 ml of chloromethyltrimethylsilane (CM-TMS; CAS Reg. No.: 2344-80-1; commercially available from Sigma-Aldrich). A solution of 14ml CM-TMS in 43ml MTBE was added slowly over 2 hours at a temperature of 55°C. The mixture was stirred at 55°C for 2 hours and then cooled to a temperature of -10°C. 10.0 g of a commercially available compound of formula (Aa) (CAS Reg. No: 51336-94-8; commercially available from Sigma-Aldrich) in 30 ml MTBE was then added and the temper...
Embodiment 2
[0311] Example 2: Preparation of wherein Y without solid extraction using HCl in THF as the second solvent 1 and Y 2 The HCl salt of the compound of formula (I) which is F
[0312] 5g of which Y obtained by Example 1 1 and Y 2The crystalline compound of formula (I) as F (16.9 mmol, cis:trans=9:1) was dissolved in 75 ml of MIBK and heated to 60°C. Then 7.2 ml of HCl in THF (23.8 mmol of HCl; concentration = 3.3 mol / l) were added in one portion. The mixture was then stirred at 60 °C for 60 min. After about 5-10 min, the mixture became cloudy and crystallized. The suspension thus obtained was cooled to ambient temperature within 90 min. Stirring was continued for another 60 min at room temperature. The solid obtained was isolated by filtration, washed twice with MIBK (2×10 ml) and dried under vacuum at 45°C.
[0313] where Y 1 and Y 2 The HCl salt of the compound of formula (I), which is F, was obtained in 78% yield as a colorless solid, corresponding to 3.95 g. where ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com